Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy
- PMID: 21784898
- PMCID: PMC3148709
- DOI: 10.1681/ASN.2010111125
Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy
Abstract
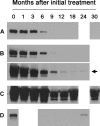
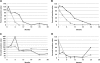
Autoantibodies to the M-type phospholipase A(2) receptor (PLA(2)R) are sensitive and specific for idiopathic membranous nephropathy. The anti-B cell agent rituximab is a promising therapy for this disease, but biomarkers of early response to treatment currently do not exist. Here, we investigated whether levels of anti-PLA(2)R correlate with the immunological activity of membranous nephropathy, potentially exhibiting a more rapid response to treatment than clinical parameters such as proteinuria. We measured the amount of anti-PLA(2)R using Western blot immunoassay in serial serum samples from a total of 35 patients treated with rituximab for membranous nephropathy in two distinct cohorts. Pretreatment samples from 25 of 35 (71%) patients contained anti-PLA(2)R, and these autoantibodies declined or disappeared in 17 (68%) of these patients within 12 months after rituximab. Those who demonstrated this immunologic response fared better clinically: 59% and 88% attained complete or partial remission by 12 and 24 months, respectively, compared with 0% and 33% among those with persistent anti-PLA(2)R levels. Changes in antibody levels preceded changes in proteinuria. One subject who relapsed during follow-up had a concomitant return of anti-PLA(2)R. In summary, measuring anti-PLA(2)R levels by immunoassay may be a method to follow and predict response to treatment with rituximab in membranous nephropathy.
Figures

Comment in
-
Circulating anti-PLA2R autoantibodies to monitor immunological activity in membranous nephropathy.J Am Soc Nephrol. 2011 Aug;22(8):1400-2. doi: 10.1681/ASN.2011060610. Epub 2011 Jul 22. J Am Soc Nephrol. 2011. PMID: 21784892 No abstract available.
References
-
- Cattran D: Management of membranous nephropathy: When and what for treatment. J Am Soc Nephrol 16: 1188–1194, 2005 - PubMed
-
- El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG: Recurrent idiopathic membranous nephropathy: Early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant 9: 2800–2807, 2009 - PubMed
-
- Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC: Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73: 117–125, 2008 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

