TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29
- PMID: 21784902
- PMCID: PMC3148701
- DOI: 10.1681/ASN.2010121308
TGF-β/Smad3 signaling promotes renal fibrosis by inhibiting miR-29
Abstract
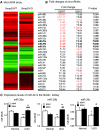
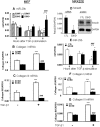
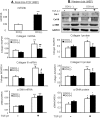
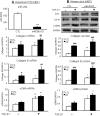
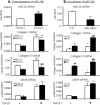
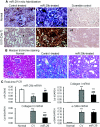
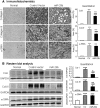
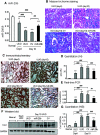
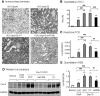
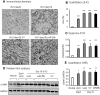
TGF-β/Smad3 signaling promotes fibrosis, but the development of therapeutic interventions involving this pathway will require the identification and ultimate targeting of downstream fibrosis-specific genes. In this study, using a microRNA microarray and real-time PCR, wild-type mice had reduced expression of miR-29 along with the development of progressive renal fibrosis in obstructive nephropathy. In contrast, Smad3 knockout mice had increased expression of miR-29 along with the absence of renal fibrosis in the same model of obstruction. In cultured fibroblasts and tubular epithelial cells, Smad3 mediated TGF-β(1)-induced downregulation of miR-29 by binding to the promoter of miR-29. Furthermore, miR-29 acted as a downstream inhibitor and therapeutic microRNA for TGF-β/Smad3-mediated fibrosis. In vitro, overexpression of miR-29b inhibited, but knockdown of miR-29 enhanced, TGF-β(1)-induced expression of collagens I and III by renal tubular cells. Ultrasound-mediated gene delivery of miR-29b either before or after established obstructive nephropathy blocked progressive renal fibrosis. In conclusion, miR-29 is a downstream inhibitor of TGF-β/Smad3-mediated fibrosis and may have therapeutic potential for diseases involving fibrosis.
Figures

Similar articles
-
The microRNA miR-433 promotes renal fibrosis by amplifying the TGF-β/Smad3-Azin1 pathway.Kidney Int. 2013 Dec;84(6):1129-44. doi: 10.1038/ki.2013.272. Epub 2013 Jul 17. Kidney Int. 2013. PMID: 23868013
-
Smad3-mediated upregulation of miR-21 promotes renal fibrosis.J Am Soc Nephrol. 2011 Sep;22(9):1668-81. doi: 10.1681/ASN.2010111168. Epub 2011 Aug 18. J Am Soc Nephrol. 2011. PMID: 21852586 Free PMC article.
-
miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-β/Smad3 signaling.Mol Ther. 2014 May;22(5):974-85. doi: 10.1038/mt.2014.25. Epub 2014 Feb 26. Mol Ther. 2014. PMID: 24569834 Free PMC article.
-
[Transforming growth factor-β and renal fibrosis].Sheng Li Xue Bao. 2018 Dec 25;70(6):612-622. Sheng Li Xue Bao. 2018. PMID: 30560270 Review. Chinese.
-
Diverse roles of TGF-β/Smads in renal fibrosis and inflammation.Int J Biol Sci. 2011;7(7):1056-67. doi: 10.7150/ijbs.7.1056. Epub 2011 Sep 2. Int J Biol Sci. 2011. PMID: 21927575 Free PMC article. Review.
Cited by
-
Modulation of miR-29a and miR-29b Expression and Their Target Genes Related to Inflammation and Renal Fibrosis by an Oral Nutritional Supplement with Probiotics in Malnourished Hemodialysis Patients.Int J Mol Sci. 2024 Jan 17;25(2):1132. doi: 10.3390/ijms25021132. Int J Mol Sci. 2024. PMID: 38256206 Free PMC article. Clinical Trial.
-
Th-MYCN mice with caspase-8 deficiency develop advanced neuroblastoma with bone marrow metastasis.Cancer Res. 2013 Jul 1;73(13):4086-97. doi: 10.1158/0008-5472.CAN-12-2681. Epub 2013 Mar 27. Cancer Res. 2013. PMID: 23536557 Free PMC article.
-
Smad3 binds Scleraxis and Mohawk and regulates tendon matrix organization.J Orthop Res. 2013 Sep;31(9):1475-83. doi: 10.1002/jor.22382. Epub 2013 May 7. J Orthop Res. 2013. PMID: 23653374 Free PMC article.
-
Diverse origins of the myofibroblast—implications for kidney fibrosis.Nat Rev Nephrol. 2015 Apr;11(4):233-44. doi: 10.1038/nrneph.2014.246. Epub 2015 Jan 13. Nat Rev Nephrol. 2015. PMID: 25584804 Review.
-
miR-155-5p Implicates in the Pathogenesis of Renal Fibrosis via Targeting SOCS1 and SOCS6.Oxid Med Cell Longev. 2020 Jun 6;2020:6263921. doi: 10.1155/2020/6263921. eCollection 2020. Oxid Med Cell Longev. 2020. PMID: 32587662 Free PMC article.
References
-
- Lan HY: Smad7 as a therapeutic agent for chronic kidney diseases. Front Biosci 13: 4984–4992, 2008 - PubMed
-
- Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC: Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 17: 19–27, 2006 - PubMed
-
- Yang F, Huang XR, Chung AC, Hou CC, Lai KN, Lan HY: Essential role for Smad3 in angiotensin II-induced tubular epithelial-mesenchymal transition. J Pathol 221: 390–401, 2010 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

