Characterization of an N-acetylmuramic acid/N-acetylglucosamine kinase of Clostridium acetobutylicum
- PMID: 21784936
- PMCID: PMC3187400
- DOI: 10.1128/JB.05514-11
Characterization of an N-acetylmuramic acid/N-acetylglucosamine kinase of Clostridium acetobutylicum
Abstract
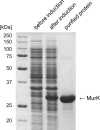
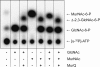
We report here the cloning and characterization of a cytoplasmic kinase of Clostridium acetobutylicum, named MurK (for murein sugar kinase). The enzyme has a unique specificity for both amino sugars of the bacterial cell wall, N-acetylmuramic acid (MurNAc) and N-acetylglucosamine (GlcNAc), which are phosphorylated at the 6-hydroxyl group. Kinetic analyses revealed Km values of 190 and 127 μM for MurNAc and GlcNAc, respectively, and a kcat value (65.0 s(-1)) that was 1.5-fold higher for the latter substrate. Neither the non-N-acetylated forms of the cell wall sugars, i.e., glucosamine and/or muramic acid, nor epimeric hexoses or 1,6-anhydro-MurNAc were substrates for the enzyme. MurK displays low overall amino acid sequence identity (24%) with human GlcNAc kinase and is the first characterized bacterial representative of the BcrAD/BadFG-like ATPase family. We propose a role of MurK in the recovery of muropeptides during cell wall rescue in C. acetobutylicum. The kinase was applied for high-sensitive detection of the amino sugars in cell wall preparations by radioactive phosphorylation.
Figures
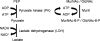


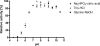

References
-
- Bethesda Research Laboratories 1986. BRL pUC host: Escherichia coli DH5α competent cells. Focus 8:9
-
- Blattner F. R., et al. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453–1462 - PubMed
-
- Bradford M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248–254 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

