Calreticulin binds to gentamicin and reduces drug-induced ototoxicity
- PMID: 21785162
- PMCID: PMC3216409
- DOI: 10.1093/toxsci/kfr196
Calreticulin binds to gentamicin and reduces drug-induced ototoxicity
Abstract
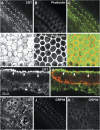
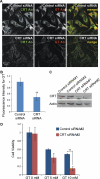
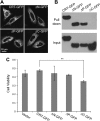
Aminoglycosides like gentamicin are among the most commonly used antibiotics in clinical practice and are essential for treating life-threatening tuberculosis and Gram-negative bacterial infections. However, aminoglycosides are also nephrotoxic and ototoxic. Although a number of mechanisms have been proposed, it is still unclear how aminoglycosides induce cell death in auditory sensory epithelia and subsequent deafness. Aminoglycosides bind to various intracellular molecules, such as RNA and phosphoinositides. We hypothesized that aminoglycosides, based on their tissue-specific susceptibility, also bind to intracellular proteins that play a role in drug-induced ototoxicity. By conjugating an aminoglycoside, gentamicin, to agarose beads and conducting a gentamicin-agarose pull-down assay, we have isolated gentamicin-binding proteins (GBPs) from immortalized cells of mouse organ of Corti, HEI-OC1. Mass spectrometry identified calreticulin (CRT) as a GBP. Immunofluorescence revealed that CRT expression is concentrated in strial marginal cells and hair cell stereocilia, primary locations of drug uptake and cytotoxicity in the cochlea. In HEI-OC1 cells treated with gentamicin, reduction of CRT expression using small interfering RNA (siRNA) reduced intracellular drug levels. CRT-deficient mouse embryonic fibroblast (MEF) cells as well as CRT siRNA-transfected wild-type MEFs also had reduced cell viability after gentamicin treatment. A pull-down assay using deletion mutants of CRT determined that the carboxyl C-domain of CRT binds to gentamicin. HeLa cells transfected with CRT C-domain deletion mutant construct were more susceptible to gentamicin-induced cytotoxicity compared with cells transfected with full-length CRT or other deletion mutants. Therefore, we conclude that CRT binding to gentamicin is protective against gentamicin-induced cytotoxicity.
Figures




Similar articles
-
Intracellular mechanisms of aminoglycoside-induced cytotoxicity.Integr Biol (Camb). 2011 Sep;3(9):879-86. doi: 10.1039/c1ib00034a. Epub 2011 Jul 29. Integr Biol (Camb). 2011. PMID: 21799993 Free PMC article. Review.
-
Sodium-glucose transporter-2 (SGLT2; SLC5A2) enhances cellular uptake of aminoglycosides.PLoS One. 2014 Sep 30;9(9):e108941. doi: 10.1371/journal.pone.0108941. eCollection 2014. PLoS One. 2014. PMID: 25268124 Free PMC article.
-
STK33 alleviates gentamicin-induced ototoxicity in cochlear hair cells and House Ear Institute-Organ of Corti 1 cells.J Cell Mol Med. 2018 Nov;22(11):5286-5299. doi: 10.1111/jcmm.13792. Epub 2018 Sep 6. J Cell Mol Med. 2018. PMID: 30256516 Free PMC article.
-
Induction of mitophagy in the HEI-OC1 auditory cell line and activation of the Atg12/LC3 pathway in the organ of Corti.Hear Res. 2018 Apr;361:52-65. doi: 10.1016/j.heares.2018.01.003. Epub 2018 Jan 11. Hear Res. 2018. PMID: 29352609
-
Aspirin attenuates gentamicin ototoxicity: from the laboratory to the clinic.Hear Res. 2007 Apr;226(1-2):178-82. doi: 10.1016/j.heares.2006.05.008. Epub 2006 Jul 17. Hear Res. 2007. PMID: 16844331 Review.
Cited by
-
Mechanisms of Ototoxicity and Otoprotection.Otolaryngol Clin North Am. 2021 Dec;54(6):1101-1115. doi: 10.1016/j.otc.2021.08.007. Otolaryngol Clin North Am. 2021. PMID: 34774227 Free PMC article. Review.
-
Intracellular mechanisms of aminoglycoside-induced cytotoxicity.Integr Biol (Camb). 2011 Sep;3(9):879-86. doi: 10.1039/c1ib00034a. Epub 2011 Jul 29. Integr Biol (Camb). 2011. PMID: 21799993 Free PMC article. Review.
-
Mechanisms of Aminoglycoside- and Cisplatin-Induced Ototoxicity.Am J Audiol. 2021 Oct 11;30(3S):887-900. doi: 10.1044/2021_AJA-21-00006. Epub 2021 Aug 20. Am J Audiol. 2021. PMID: 34415784 Free PMC article. Review.
-
Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies.Cold Spring Harb Perspect Med. 2019 Nov 1;9(11):a033548. doi: 10.1101/cshperspect.a033548. Cold Spring Harb Perspect Med. 2019. PMID: 30559254 Free PMC article. Review.
-
Unscrambling exit site patterns on the endoplasmic reticulum as a quenched demixing process.Biophys J. 2021 Jun 15;120(12):2532-2542. doi: 10.1016/j.bpj.2021.04.023. Epub 2021 Apr 29. Biophys J. 2021. PMID: 33932435 Free PMC article.
References
-
- Bastianutto C, Clementi E, Codazzi F, Podini P, De Giorgi F, Rizzuto R, Meldolesi J, Pozzan T. Overexpression of calreticulin increases the Ca2+ capacity of rapidly exchanging Ca2+ stores and reveals aspects of their lumenal microenvironment and function. J. Cell Biol. 1995;130:847–855. - PMC - PubMed
-
- Chu HQ, Xiong H, Zhou XQ, Han F, Wu ZG, Zhang P, Huang XW, Cui YH. Aminoglycoside ototoxicity in three murine strains and effects on NKCC1 of stria vascularis. Chin. Med. J. (Engl) 2006;119:980–985. - PubMed
-
- Coling DE, Ding D, Young R, Lis M, Stofko E, Blumenthal KM, Salvi RJ. Proteomic analysis of cisplatin-induced cochlear damage: Methods and early changes in protein expression. Hear. Res. 2007;226:140–156. - PubMed
-
- Edson RS, Terrell CL. The aminoglycosides. Mayo Clin. Proc. 1999;74:519–528. - PubMed
-
- Fliegel L, Burns K, MacLennan DH, Reithmeier RA, Michalak M. Molecular cloning of the high affinity calcium-binding protein (calreticulin) of skeletal muscle sarcoplasmic reticulum. J. Biol. Chem. 1989;264:21522–21528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

