Shock and awe: unleashing the heat shock response to treat Huntington disease
- PMID: 21785212
- PMCID: PMC3148752
- DOI: 10.1172/JCI59190
Shock and awe: unleashing the heat shock response to treat Huntington disease
Abstract
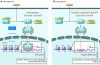
The heat shock response (HSR) is a highly conserved protective mechanism that enables cells to withstand diverse environmental stressors that disrupt protein homeostasis (proteostasis) and promote protein misfolding. It has been suggested that small-molecule drugs that elicit the HSR by activating the transcription factor heat shock factor 1 might help mitigate protein misfolding and aggregation in several devastating neurodegenerative disorders, including Huntington disease (HD). In this issue of the JCI, Labbadia et al. use a brain-penetrant Hsp90 inhibitor, HSP990, to induce the HSR in mouse models of HD. Unexpectedly, they observed that HSP990 confers only transient amelioration of a subset of HD-related phenotypes, because alterations in chromatin architecture impair the HSR upon disease progression. These findings suggest that synergistic combination therapies that simultaneously unleash the HSR and prevent its impairment are likely to be needed to restore proteostasis in HD.
Figures
Comment on
-
Altered chromatin architecture underlies progressive impairment of the heat shock response in mouse models of Huntington disease.J Clin Invest. 2011 Aug;121(8):3306-19. doi: 10.1172/JCI57413. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785217 Free PMC article.
References
-
- Scherzinger E, et al. Huntingtin-encoded polyglutamine expansions form amyloid-like protein aggregates in vitro and in vivo. Cell. 1997;90(3):549–558. - PubMed

