Prolactin increases SMN expression and survival in a mouse model of severe spinal muscular atrophy via the STAT5 pathway
- PMID: 21785216
- PMCID: PMC3148738
- DOI: 10.1172/JCI46276
Prolactin increases SMN expression and survival in a mouse model of severe spinal muscular atrophy via the STAT5 pathway
Erratum in
- J Clin Invest. 2011 Sep 1;121(9):3763
Abstract
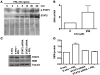
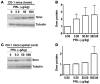
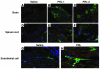
Spinal muscular atrophy (SMA) is an autosomal recessive neurodegenerative disease that is characterized by the loss of motor neurons, resulting in progressive muscle atrophy. It is caused by the loss of functional survival motor neuron (SMN) protein due to mutations or deletion in the SMN1 gene. A potential treatment strategy for SMA is to upregulate levels of SMN protein. Several agents that activate STAT5 in human and mouse cell lines enhance SMN expression from the SMN2 gene and can compensate, at least in part, for the loss of production of a functional protein from SMN1. Here, we have shown that prolactin (PRL) increases SMN levels via activation of the STAT5 pathway. PRL increased SMN mRNA and protein levels in cultured human and mouse neuronal cells. Administration of STAT5-specific siRNA blocked the effects of PRL, indicating that the PRL-induced transcriptional upregulation of the SMN-encoding gene was mediated by activation of STAT5. Furthermore, systemic administration of PRL to WT mice induced SMN expression in the brain and spinal cord. Critically, PRL treatment increased SMN levels, improved motor function, and enhanced survival in a mouse model of severe SMA. Our results confirm earlier work suggesting STAT5 pathway activators as potential therapeutic compounds for the treatment of SMA and identify PRL as one such promising agent.
Figures
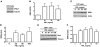
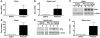
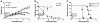
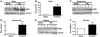

Comment in
-
Of SMN in mice and men: a therapeutic opportunity.J Clin Invest. 2011 Aug;121(8):2978-81. doi: 10.1172/JCI58752. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785213 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

