Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies
- PMID: 21785218
- PMCID: PMC3148747
- DOI: 10.1172/JCI57834
Limited efficacy of inactivated influenza vaccine in elderly individuals is associated with decreased production of vaccine-specific antibodies
Abstract
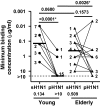
During seasonal influenza epidemics, disease burden is shouldered predominantly by the very young and the elderly. Elderly individuals are particularly affected, in part because vaccine efficacy wanes with age. This has been linked to a reduced ability to induce a robust serum antibody response. Here, we show that this is due to reduced quantities of vaccine-specific antibodies, rather than a lack of antibody avidity or affinity. We measured levels of vaccine-specific plasmablasts by ELISPOT 1 week after immunization of young and elderly adults with inactivated seasonal influenza vaccine. Plasmablast-derived polyclonal antibodies (PPAbs) were generated from bulk-cultured B cells, while recombinant monoclonal antibodies (re-mAbs) were produced from single plasmablasts. The frequency of vaccine-specific plasmablasts and the concentration of PPAbs were lower in the elderly than in young adults, whereas the yields of secreted IgG per plasmablast were not different. Differences were not detected in the overall vaccine-specific avidity or affinity of PPAbs and re-mAbs between the 2 age groups. In contrast, reactivity of the antibodies induced by the inactivated seasonal influenza vaccine toward the 2009 pandemic H1N1 virus, which was not present in the vaccine, was higher in the elderly than in the young. These results indicate that the inferior antibody response to influenza vaccination in the elderly is primarily due to reduced quantities of vaccine-specific antibodies. They also suggest that exposure history affects the cross-reactivity of vaccination-induced antibodies.
Figures
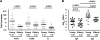
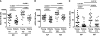
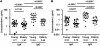

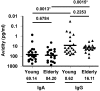

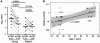
Comment in
-
Quantity, not quality, of antibody response decreased in the elderly.J Clin Invest. 2011 Aug;121(8):2981-3. doi: 10.1172/JCI58406. Epub 2011 Jul 25. J Clin Invest. 2011. PMID: 21785210 Free PMC article.
References
-
- Fiore AE, et al. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP), 2007. MMWR Recomm Rep. 2007;56(RR-6):1–54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI057266/AI/NIAID NIH HHS/United States
- U19 AI090023/AI/NIAID NIH HHS/United States
- U19 AI057229/AI/NIAID NIH HHS/United States
- U19 AI090019/AI/NIAID NIH HHS/United States
- DK56339/DK/NIDDK NIH HHS/United States
- U54 AI057158/AI/NIAID NIH HHS/United States
- P30 DK056339/DK/NIDDK NIH HHS/United States
- AI057229/AI/NIAID NIH HHS/United States
- P30 AR053483/AR/NIAMS NIH HHS/United States
- U19 AI057266/AI/NIAID NIH HHS/United States
- HHSN266200500026C/AI/NIAID NIH HHS/United States
- AI057158/AI/NIAID NIH HHS/United States
- AI090023/AI/NIAID NIH HHS/United States

