Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication
- PMID: 21785410
- PMCID: PMC3160662
- DOI: 10.1038/emboj.2011.242
Targeting of Nbp1 to the inner nuclear membrane is essential for spindle pole body duplication
Abstract
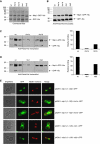
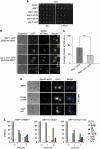
Spindle pole bodies (SPBs), like nuclear pore complexes, are embedded in the nuclear envelope (NE) at sites of fusion of the inner and outer nuclear membranes. A network of interacting proteins is required to insert a cytoplasmic SPB precursor into the NE. A central player of this network is Nbp1 that interacts with the conserved integral membrane protein Ndc1. Here, we establish that Nbp1 is a monotopic membrane protein that is essential for SPB insertion at the inner face of the NE. In vitro and in vivo studies identified an N-terminal amphipathic α-helix of Nbp1 as a membrane-binding element, with crucial functions in SPB duplication. The karyopherin Kap123 binds to a nuclear localization sequence next to this amphipathic α-helix and prevents unspecific tethering of Nbp1 to membranes. After transport into the nucleus, Nbp1 binds to the inner nuclear membrane. These data define the targeting pathway of a SPB component and suggest that the amphipathic α-helix of Nbp1 is important for SPB insertion into the NE from within the nucleus.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures






References
-
- Adams IR, Kilmartin JV (2000) Spindle pole body duplication: a model for centrosome duplication? Trends Cell Biol 10: 329–335 - PubMed
-
- Alber F, Dokudovskaya S, Veenhoff LM, Zhang W, Kipper J, Devos D, Suprapto A, Karni-Schmidt O, Williams R, Chait BT, Sali A, Rout MP (2007) The molecular architecture of the nuclear pore complex. Nature 450: 695–701 - PubMed
-
- Antonin W (2009) Nuclear envelope: membrane bending for pore formation? Curr Biol 19: R410–R412 - PubMed
-
- Araki Y, Lau CK, Maekawa H, Jaspersen SL, Giddings TH Jr, Schiebel E, Winey M (2006) The Saccharomyces cerevisiae spindle pole body (SPB) component Nbp1p is required for SPB membrane insertion and interacts with the integral membrane proteins Ndc1p and Mps2p. Mol Biol Cell 17: 1959–1970 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

