Efficient induction of a Her2-specific anti-tumor response by dendritic cells pulsed with a Hsp70L1-Her2(341-456) fusion protein
- PMID: 21785448
- PMCID: PMC4012883
- DOI: 10.1038/cmi.2011.21
Efficient induction of a Her2-specific anti-tumor response by dendritic cells pulsed with a Hsp70L1-Her2(341-456) fusion protein
Abstract
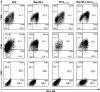
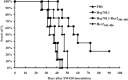
Heat shock proteins (HSPs) have been shown to interact with antigen-presenting cells (APCs), especially dendritic cells (DCs). HSPs act as potent adjuvants, inducing a Th1 response, as well as antigen-specific CD8(+) cytotoxic T lymphocytes (CTL) via cross-presentation. Our previous work has demonstrated that Hsp70-like protein 1 (Hsp70L1), a new member of the Hsp70 subfamily, can act as a powerful Th1 adjuvant in a DC-based vaccine. Here we report the efficient induction of tumor antigen-specific T cell immune response by DCs pulsed with recombinant fusion protein of Hsp70L1 and Her2(341-456), the latter of which is a fragment of Her2/neu (Her2) containing E75 (a HLA-A2 restricted CTL epitope). The fusion protein Hsp70L1-Her2(341-456) promotes the maturation of DCs and activates them to produce cytokines, such as IL-12 and TNF-α, and chemokines, such as MIP-1α, MIP-1β and RANTES. Taken together, these results indicate that the adjuvant activity of Hsp70L1 is maintained in the fusion protein. Her2-specific HLA-A2.1-restricted CD8(+) CTLs can be generated efficiently either from the Peripheral blood lymphocytes (PBL) of healthy donors or from the splenocytes of immunized HLA-A2.1/K(b) transgenic mice by in vitro stimulation or immunization with DCs pulsed with the Hsp70L1-Her2(341-456) fusion protein. This results in more potent target cell killing in an antigen-specific and HLA-A2.1-restricted manner. Adoptive transfer of splenocytes from transgenic mice immunized with Hsp70L1-Her2(341-456)-pulsed DCs can markedly inhibit tumor growth and prolong the survival of nude mice with Her2(+)/HLA-A2.1(+) human carcinomas. These results suggest that Hsp70L1-Her2(341-456)-pulsed DCs could be a new therapeutic vaccine for patients with Her2(+) cancer.
Figures




Similar articles
-
HSP70L1-mediated intracellular priming of dendritic cell vaccination induces more potent CTL response against cancer.Cell Mol Immunol. 2018 Feb;15(2):135-145. doi: 10.1038/cmi.2016.33. Epub 2016 Jun 27. Cell Mol Immunol. 2018. PMID: 27345726 Free PMC article.
-
Hsp70-like protein 1 fusion protein enhances induction of carcinoembryonic antigen-specific CD8+ CTL response by dendritic cell vaccine.Cancer Res. 2005 Jun 1;65(11):4947-54. doi: 10.1158/0008-5472.CAN-04-3912. Cancer Res. 2005. PMID: 15930317
-
Novel heat shock protein Hsp70L1 activates dendritic cells and acts as a Th1 polarizing adjuvant.Blood. 2004 Mar 1;103(5):1747-54. doi: 10.1182/blood-2003-08-2828. Epub 2003 Oct 30. Blood. 2004. PMID: 14592822
-
Immunostimulatory DNA sequences and cancer therapy.Springer Semin Immunopathol. 2000;22(1-2):107-16. doi: 10.1007/s002810000022. Springer Semin Immunopathol. 2000. PMID: 10944805 Review. No abstract available.
-
Cancer vaccine strategies: translation from mice to human clinical trials.Cancer Immunol Immunother. 2018 Dec;67(12):1863-1869. doi: 10.1007/s00262-017-2084-x. Epub 2017 Nov 15. Cancer Immunol Immunother. 2018. PMID: 29143114 Free PMC article. Review.
Cited by
-
Intracellular HSP70L1 inhibits human dendritic cell maturation by promoting suppressive H3K27me3 and H2AK119Ub1 histone modifications.Cell Mol Immunol. 2020 Jan;17(1):85-94. doi: 10.1038/s41423-018-0195-8. Epub 2019 Jan 11. Cell Mol Immunol. 2020. PMID: 30635648 Free PMC article.
-
Intercepting Premalignant, Preinvasive Breast Lesions Through Vaccination.Front Immunol. 2021 Nov 24;12:786286. doi: 10.3389/fimmu.2021.786286. eCollection 2021. Front Immunol. 2021. PMID: 34899753 Free PMC article.
-
Dendritic Cell-Based Cancer Immunotherapy against Multiple Myeloma: From Bench to Clinic.Chonnam Med J. 2015 Apr;51(1):1-7. doi: 10.4068/cmj.2015.51.1.1. Epub 2015 Apr 14. Chonnam Med J. 2015. PMID: 25914874 Free PMC article. Review.
-
Dendritic cells modified by tumor associated antigen SMP30 have enhanced antitumor effect against mouse hepatocarcinoma cells in vitro and in vivo.Am J Transl Res. 2022 Aug 15;14(8):5785-5799. eCollection 2022. Am J Transl Res. 2022. PMID: 36105050 Free PMC article.
-
HSP70L1-mediated intracellular priming of dendritic cell vaccination induces more potent CTL response against cancer.Cell Mol Immunol. 2018 Feb;15(2):135-145. doi: 10.1038/cmi.2016.33. Epub 2016 Jun 27. Cell Mol Immunol. 2018. PMID: 27345726 Free PMC article.
References
-
- Srivastava PK. Therapeutic cancer vaccines. Curr Opin Immunol. 2006;18:201–205. - PubMed
-
- Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccines. 2008;7:1185–1199. - PubMed
-
- Srivastava PK. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. - PubMed
-
- Enomoto Y, Bharti A, Khaleque AA, Song B, Liu C, Apostolopoulos V, et al. Enhanced immunogenicity of heat shock protein 70 peptide complexes from dendritic cell–tumor fusion cells. J Immunol. 2006;177:5946–5955. - PubMed
-
- Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor derived heat shock protein preparations. Science. 1997;278:117–120. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

