Design and Implementation of Functional Nanoelectronic Interfaces With Biomolecules, Cells, and Tissue Using Nanowire Device Arrays
- PMID: 21785576
- PMCID: PMC3140208
- DOI: 10.1109/TNANO.2009.2031807
Design and Implementation of Functional Nanoelectronic Interfaces With Biomolecules, Cells, and Tissue Using Nanowire Device Arrays
Abstract
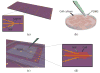
Nanowire FETs (NWFETs) are promising building blocks for nanoscale bioelectronic interfaces with cells and tissue since they are known to exhibit exquisite sensitivity in the context of chemical and biological detection, and have the potential to form strongly coupled interfaces with cell membranes. We present a general scheme that can be used to assemble NWs with rationally designed composition and geometry on either planar inorganic or biocompatible flexible plastic surfaces. We demonstrate that these devices can be used to measure signals from neurons, cardiomyocytes, and heart tissue. Reported signals are in millivolts range, which are equal to or substantially greater than those recorded with either planar FETs or multielectrode arrays, and demonstrate one unique advantage of NW-based devices. Basic studies showing the effect of device sensitivity and cell/substrate junction quality on signal magnitude are presented. Finally, our demonstrated ability to design high-density arrays of NWFETs enables us to map signal at the subcellular level, a functionality not enabled by conventional microfabricated devices. These advances could have broad applications in high-throughput drug assays, fundamental biophysical studies of cellular function, and development of powerful prosthetics.
Figures


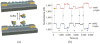










References
-
- Hille B. Ion Channels of Excitable Membranes. Sunderland, MA: Sinauer; 2001.
-
- Zipes DP, Jalife J. Cardiac Electrophysiology: From Cell to Bedside. Philadelphia, PA: Saunders; 2004. pp. 169–253.
-
- Dhein S, Mohr FW, Delmar M. Practical Methods in Cardiovascular Research. Berlin, Germany: Springer-Verlag; 2005. pp. 215–453.
-
- Zochowski M, Wachowiak M, Falk CX, Cohen LB, Lam YW, Antic S, Zecevic D. Concepts in imaging and microscopy - Imaging membrane potential with voltage-sensitive dyes. Biol Bull. 2000;198(1):1–12. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
