Inhalation of ortho-phthalaldehyde vapor causes respiratory sensitization in mice
- PMID: 21785612
- PMCID: PMC3137992
- DOI: 10.1155/2011/751052
Inhalation of ortho-phthalaldehyde vapor causes respiratory sensitization in mice
Abstract
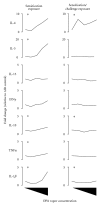
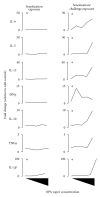
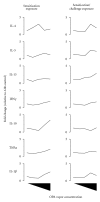
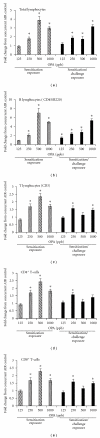
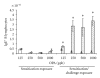
Ortho-Phthalaldehyde (OPA) has been approved for high-level sterilization of heat-sensitive medical instruments and is increasingly being used as a replacement in the healthcare industry for glutaraldehyde, a known sensitizer. Numerous case reports have been published indicating workers and patients experiencing respiratory problems, anaphylaxis, skin reactivity, and systemic antibody production. Our laboratory previously demonstrated that OPA is a dermal sensitizer in mice. The goal of the present study was to determine if OPA is a respiratory sensitizer following inhalation exposure. Mice were exposed to OPA vapor and airway and lymph nodes were examined for cytokine gene expression and alterations in lymphocyte populations. Inhalation of OPA for 3 days resulted in a concentration-dependent increase in lymphocyte proliferation, mainly B lymphocytes, in the draining lymph nodes. A secondary challenge of mice with OPA resulted in a dramatic increase in the population of B lymphocytes expressing IgE. Expression of Th2 (IL-4, IL-5, and IL-13) and anti/proinflammatory (IL-10, TNFα, and IL-1β) cytokine genes was upregulated in the lymph nodes and the nasal mucosa. Mice exposed to the higher concentrations of OPA-produced OPA-specific IgG(1) antibodies indicating systemic sensitization. These findings provide evidence that OPA has the potential to cause respiratory sensitization in mice.
Figures

References
-
- Curran AD, Burge PS, Wiley K. Clinical and immunologic evaluation of workers exposed to glutaraldehyde. Allergy. 1996;51(11):826–832. - PubMed
-
- Di Stefano F, Siriruttanapruk S, McCoach J, Burge PS. Glutaraldehyde: an occupational hazard in the hospital setting. Allergy. 1999;54(10):1105–1109. - PubMed
-
- Di Stefano F, Siriruttanapruk S, McCoach JS, Burge PS. Occupational asthma due to glutaraldehyde. Monaldi Archives for Chest Disease. 1998;53:50–55. - PubMed
-
- Maier LE, Lampel HP, Bhutani T, Jacob SE. Hand dermatitis: a focus on allergic contact dermatitis to biocides. Dermatologic Clinics. 2009;27(3):251–264. - PubMed
LinkOut - more resources
Full Text Sources

