SPECT Imaging of Epilepsy: An Overview and Comparison with F-18 FDG PET
- PMID: 21785722
- PMCID: PMC3139140
- DOI: 10.1155/2011/813028
SPECT Imaging of Epilepsy: An Overview and Comparison with F-18 FDG PET
Abstract
Epilepsy surgery is highly effective in treating refractory epilepsy, but requires accurate presurgical localization of the epileptogenic focus. Briefly, localization of the region of seizure onset traditionally dependents on seizure semiology, scalp EEG recordings and correlation with anatomical imaging modalities such as MRI. The introduction of noninvasive functional neuroimaging methods, including single-photon emission computed tomography (SPECT) and positron emission tomography (PET) has dramatically changed the method for presurgical epilepsy evaluation. These imaging modalities have become powerful tools for the investigation of brain function and are an essential part of the evaluation of epileptic patients. Of these methods, SPECT has the practical capacity to image blood flow functional changes that occur during seizures in the routine clinical setting. In this review we present the basic principles of epilepsy SPECT and PET imaging. We discuss the properties of the SPECT tracers to be used for this purpose and imaging acquisition protocols as well as the diagnostic performance of SPECT in addition to SPECT image analysis methods. This is followed by a discussion and comparison to F-18 FDG PET acquisition and imaging analysis methods.
Figures

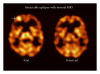

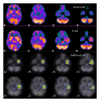
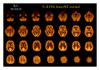
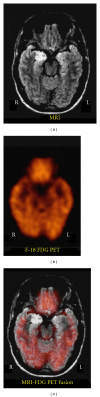
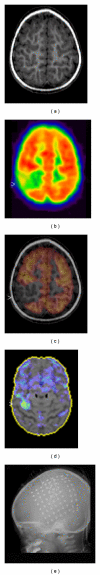
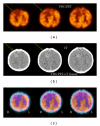
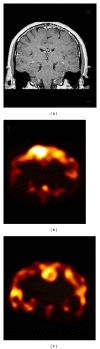
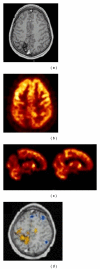
References
-
- Engel J., Jr. Outcome with respect to epileptic seizures. In: Engel J Jr., editor. Surgical Treatment of the Epilepsies. New York, NY, USA: Raven Press; 1987. pp. 553–571.
-
- Holman BL, Devous MD. Functional brain SPECT: the emergence of a powerful clinical method. Journal of Nuclear Medicine. 1992;33(10):1888–1904. - PubMed
-
- Lassen NA, Blasberg RG. Technetium-99m-d,l-HM-PAO, the development of a new class of Tc-labeled tracers: an overview. Journal of Cerebral Blood Flow and Metabolism. 1988;8(6):S1–S3. - PubMed
-
- Walovitch RC, Hill TC, Garrity ST, et al. Characterization of technetium-99m-L,L-ECD for brain perfusion imaging, part 1: pharmacology of technetium-99m ECD in nonhuman primates. Journal of Nuclear Medicine. 1989;30(11):1892–1901. - PubMed
-
- Deutsch G, Mountz JM. Neuroimaging evidence of diaschisis and reorganization in stroke recovery. In: Christensen AL, Uzzell B, editors. International Handbook of Neuropsychological Rehabilitation. New York, NY, USA: Plenum; 2000. pp. 33–63.
LinkOut - more resources
Full Text Sources

