Evaluation of autologous platelet concentrate for intertransverse lumbar fusion
- PMID: 21786039
- PMCID: PMC3175828
- DOI: 10.1007/s00586-011-1904-5
Evaluation of autologous platelet concentrate for intertransverse lumbar fusion
Abstract
Introduction: The aim of the study was to analyze if the adding of autologous platelet concentrate (APC) to a mixture of local autograft plus tricalcium phosphate and hidroxiapatite (TCP/HA) would improve the fusion rate in posterolateral lumbar fusion.
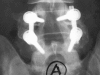
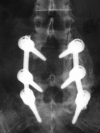
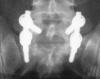
Materials and methods: A prospective, controlled, blinded, non-randomized clinical trial was carried out in 107 patients affected by degenerative lumbar pathology. The study group consisted of 67 patients, in which autologous platelet concentration was added to a mixture of autologous local bone graft and TCP/HA. A control group of 40 patients with same pathology and surgical technique but without APC addition was used to compare the fusion mass obtained. By means of plain X-rays, a blinded evaluation of the intertransverse fusion mass quality at twelve and twenty-four months was made according to type A (bilateral uniform mass), type B (unilateral uniform mass) and type C (irregular or lack bilateral mass). Patients with type C were regarded as pseudoarthrosis.
Results: In the study group 17 patients had lack or irregular fusion mass (25.4%) versus three patients in the control group (7.5%), which was statistically significant.
Conclusions: This study shows that the adding of autologous platelet concentration to a mixture of autologous bone graft plus TCP/HA has decreased our rates of posterolateral lumbar fusion.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical