Fluid shear stress alters the hemostatic properties of endothelial outgrowth cells
- PMID: 21787250
- PMCID: PMC3246409
- DOI: 10.1089/ten.TEA.2010.0290
Fluid shear stress alters the hemostatic properties of endothelial outgrowth cells
Abstract
Surface endothelialization is an attractive means to improve the performance of small diameter vascular grafts. While endothelial outgrowth cells (EOCs) are considered a promising source of autologous endothelium, the ability of EOCs to modulate coagulation-related blood activities is not well understood. The goal of this study was to assess the role of arterial flow conditions on the thrombogenic phenotype of EOCs. EOCs derived from baboon peripheral blood, as well as mature arterial endothelial cells from baboons, were seeded onto adsorbed collagen, then exposed to physiologic levels of fluid shear stress. For important hemostatic pathways, cellular responses to shear stress were characterized at the gene and protein level and confirmed with a functional assay for activated protein C (APC) activity. For EOCs, fluid shear stress upregulated gene and protein expression of anticoagulant and platelet inhibitory factors, including thrombomodulin, tissue factor pathway inhibitor, and nitric oxide synthase 3 (eNOS). Fluid shear stress significantly altered the functional activity of EOCs by increasing APC levels. This study demonstrates that fluid shear stress is an important determinant of EOC hemostatic properties. Accordingly, manipulation of EOC phenotype by mechanical forces may be important for the development of thrombo-resistant surfaces on engineered vascular implants.
Figures
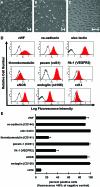
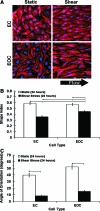


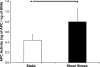
References
-
- Meinhart J.G. Deutsch M. Fischlein T. Howanietz N. Froschl A. Zilla P. Clinical autologous in vitro endothelialization of 153 infrainguinal eptfe grafts. Ann Thorac Surg. 2001;71:S327. - PubMed
-
- Deutsch M. Meinhart J. Zilla P. Howanietz N. Gorlitzer M. Froeschl A. Stuempflen A. Bezuidenhout D. Grabenwoeger M. Long-term experience in autologous in vitro endothelialization of infrainguinal eptfe grafts. J Vasc Surg. 49:352. discussion 362, 2009. - PubMed
-
- Hinds M.T. Ma M. Tran N. Ensley A.E. Kladakis S.M. Vartanian K.B. Markway B.D. Nerem R.M. Hanson S.R. Potential of baboon endothelial progenitor cells for tissue engineered vascular grafts. J Biomed Mater Res A. 2008;86:804. - PubMed
-
- Melero-Martin J.M. Khan Z.A. Picard A. Wu X. Paruchuri S. Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

