Inferior quantitative and qualitative immune responses to pneumococcal conjugate vaccine in infants with nasopharyngeal colonization by Streptococcus pneumoniae during the primary series of immunization
- PMID: 21787822
- PMCID: PMC3167924
- DOI: 10.1016/j.vaccine.2011.07.035
Inferior quantitative and qualitative immune responses to pneumococcal conjugate vaccine in infants with nasopharyngeal colonization by Streptococcus pneumoniae during the primary series of immunization
Abstract
Background: Heightened immunogenicity, measured one month after the primary series of pneumococcal conjugate vaccine (PCV), in African children was previously hypothesized to be due to increased rates of nasopharyngeal pneumococcal colonization during early infancy.
Methods: We analyzed the effect of selected vaccine-serotype (6B, 19F and 23F) nasopharyngeal colonization prior to the first PCV dose or when colonized for the first time prior to the second or third (2nd/3rd) PCV dose on serotype quantitative and qualitative antibody responses.
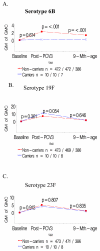
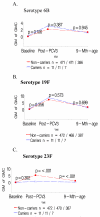
Results: Colonization prior to receiving the first PCV was associated with lower geometric mean antibody concentrations (GMCs) one month after the third dose of PCV and six months later to the colonizing-serotype. Colonized infants also had lower geometric mean titers (GMTs) on opsonophagocytosis activity assay (OPA) and a lower proportion had titers ≥ 8 against the colonizing serotypes (19F and 23F) post vaccination. Colonization occurring only prior to the 2nd/3rdPCV dose was also associated with lower GMCs and OPA GMTs to the colonizing-serotype. The effect of colonization with serotypes 19F and 23F prior to PCV vaccination had a greater effect on a lower proportion of colonized infants having OPA titers ≥ 8 than the effect of colonization on the lower proportion with antibody ≥ 0.35 μg/ml.
Conclusion: Infant nasopharyngeal colonization at any stage before completing the primary series of PCV vaccination was associated with inferior quantitative and qualitative antibody responses to the colonizing-serotype.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures




References
-
- O’Brien KL, Dagan R. The potential indirect effect of conjugate pneumococcal vaccines. Vaccine. 2003 May 16;21(17-18):1815–25. - PubMed
-
- Mbelle N, Huebner RE, Wasas AD, Kimura A, Chang I, Klugman KP. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J Infect Dis. 1999;180(4):1171–6. 1999. - PubMed
-
- Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen JR, et al. Northern California Kaiser Permanente Vaccine Study Center Group Efficacy, safety and immunogenicity of heptavalent pneumococcal conjugate vaccine in children. Pediatr Infect Dis J. 2000 Mar;19(3):187–95. - PubMed
-
- Nurkka A, Ahman H, Korkeila M, Jantti V, Kayhty H, Eskola J. Serum and salivary anti-capsular antibodies in infants and children immunized with the heptavalent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2001;20(1):25–33. - PubMed
-
- Obaro SK, Adegbola RA, Chang I, Banya WA, Jaffar S, Mcadam KW, et al. Safety and immunogenicity of a nonavalent pneumococcal vaccine conjugated to CRM197 administered simultaneously but in a separate syringe with diphtheria, tetanus and pertussis vaccines in Gambian infants. Pediatr Infect Dis J. 2000;19(5):463–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

