Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus
- PMID: 21787867
- PMCID: PMC3166433
- DOI: 10.1016/j.neuroimage.2011.07.003
Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus
Abstract
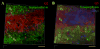
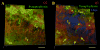
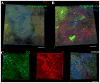
Neuronal M-channels are low threshold, slowly activating and non-inactivating, voltage dependent K(+) channels that play a crucial role in controlling neuronal excitability. The native M-channel is composed of heteromeric or homomeric assemblies of subunits belonging to the Kv7/KCNQ family, with KCNQ2/3 heteromers being the most abundant form. KCNQ2 and KCNQ3 subunits have been found to be expressed in various neurons in the central and peripheral nervous system of rodents and humans. Previous evidence shows preferential localization of both subunits to axon initial segments, somata and nodes of Ranvier. In this work, we show the distribution and co-localization of KCNQ2 and KCNQ3 subunits throughout the hippocampal formation, via immunostaining experiments on unfixed rat brain slices and confocal microscopy. We find intense localization and colocalization to the axonal initial segment in several regions of the hippocampus, as well as staining for non-neuronal cells in the area of the lateral ventricle. We did not observe colocalization of KCNQ2 or KCNQ3 with the presynaptic protein, synaptophysin.
Copyright © 2011 Elsevier Inc. All rights reserved.
Figures



References
-
- Amaral DG, Witter MP. Hippocampal Formation. The Rat Nervous System. 1995:443–493.
-
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. - PubMed
-
- Boscia F, Annunziato L, Taglialatela M. Retigabine and flupirtine exert neuroprotective actions in organotypic hippocampal cultures. Neuropharmacology. 2006;51:283–294. - PubMed
-
- Brown BS, Yu SP. Modulation and genetic identification of the M channel. Prog Biophys Mol Biol. 2000;73:135–166. - PubMed
-
- Brown DA, Adams PR. Muscarinic suppression of a novel voltage-sensitive K+ current in a vertebrate neurone. Nature. 1980;283:673–676. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

