Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia
- PMID: 21788367
- PMCID: PMC3144415
- DOI: 10.1083/jcb.201102076
Septin GTPases spatially guide microtubule organization and plus end dynamics in polarizing epithelia
Abstract
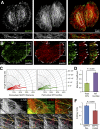
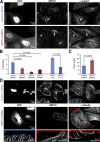
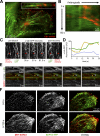
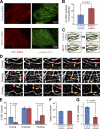
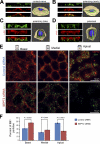
Establishment of epithelial polarity requires the reorganization of the microtubule (MT) cytoskeleton from a radial array into a network positioned along the apicobasal axis of the cell. Little is known about the mechanisms that spatially guide the remodeling of MTs during epithelial polarization. Septins are filamentous guanine triphosphatases (GTPases) that associate with MTs, but the function of septins in MT organization and dynamics is poorly understood. In this paper, we show that in polarizing epithelia, septins guide the directionality of MT plus end movement by suppressing MT catastrophe. By enabling persistent MT growth, two spatially distinct populations of septins, perinuclear and peripheral filaments, steer the growth and capture of MT plus ends. This navigation mechanism is essential for the maintenance of perinuclear MT bundles and for the orientation of peripheral MTs as well as for the apicobasal positioning of MTs. Our results suggest that septins provide the directional guidance cues necessary for polarizing the epithelial MT network.
Figures
References
-
- Applewhite D.A., Grode K.D., Keller D., Zadeh A.D., Slep K.C., Rogers S.L. 2010. The spectraplakin Short stop is an actin-microtubule cross-linker that contributes to organization of the microtubule network. Mol. Biol. Cell. 21:1714–1724 (published erratum appears in Mol. Biol. Cell.2010. 21:2097) 10.1091/mbc.E10-01-0011 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

