Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis
- PMID: 21788439
- PMCID: PMC3167080
- DOI: 10.4049/jimmunol.1003952
Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis
Abstract
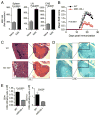
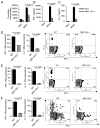
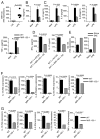
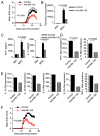
IFN-γ-producing Th1 and IL-17-producing Th17 cells are the key participants in various autoimmune diseases, including multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE). Although both of these T cell subsets are known to be regulated by specific transcription factors and cytokines, the role of microRNAs that control these two inflammatory T cell subsets and whether targeting microRNAs can have therapeutic effects are not known. In this study, we show that microRNA-155 (Mir-155) expression is elevated in CD4(+) T cells during EAE, and Mir-155(-/-) mice had a delayed course and reduced severity of disease and less inflammation in the CNS. The attenuation of EAE in Mir-155(-/-) mice was associated with a decrease in Th1 and Th17 responses in the CNS and peripheral lymphoid organs. The T cell-intrinsic function of Mir-155(-/-) was demonstrated by the resistance of Mir-155(-/-) CD4(+) T cell-repleted Rag-1(-/-) mice to EAE. Finally, we found that anti-Mir-155 treatment reduced clinical severity of EAE when given before and after the appearance of clinical symptoms. These findings demonstrate that Mir-155 confers susceptibility to EAE by affecting inflammatory T cell responses and identify Mir-155 as a new target for therapeutic intervention in multiple sclerosis.
Conflict of interest statement
The authors have no financial conflicts of interest.
Figures


References
-
- Diveu C, McGeachy MJ, Cua DJ. Cytokines that regulate autoimmunity. Curr Opin Immunol. 2008;20:663–668. - PubMed
-
- Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat Med. 2002;8:500–508. - PubMed
-
- Kebir H, Ifergan I, Alvarez JI, Bernard M, Poirier J, Arbour N, Duquette P, Prat A. Preferential recruitment of interferon-gamma-expressing TH17 cells in multiple sclerosis. Ann Neurol. 2009;66:390–402. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

