Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis
- PMID: 21788462
- PMCID: PMC3187015
- DOI: 10.1128/AAC.00366-11
Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis
Abstract
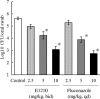
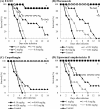
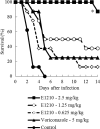
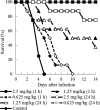
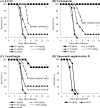
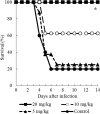
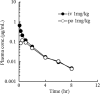
E1210 is a first-in-class, broad-spectrum antifungal with a novel mechanism of action-inhibition of fungal glycosylphosphatidylinositol biosynthesis. In this study, the efficacies of E1210 and reference antifungals were evaluated in murine models of oropharyngeal and disseminated candidiasis, pulmonary aspergillosis, and disseminated fusariosis. Oral E1210 demonstrated dose-dependent efficacy in infections caused by Candida species, Aspergillus spp., and Fusarium solani. In the treatment of oropharyngeal candidiasis, E1210 and fluconazole each caused a significantly greater reduction in the number of oral CFU than the control treatment (P < 0.05). In the disseminated candidiasis model, mice treated with E1210, fluconazole, caspofungin, or liposomal amphotericin B showed significantly higher survival rates than the control mice (P < 0.05). E1210 was also highly effective in treating disseminated candidiasis caused by azole-resistant Candida albicans or Candida tropicalis. A 24-h delay in treatment onset minimally affected the efficacy outcome of E1210 in the treatment of disseminated candidiasis. In the Aspergillus flavus pulmonary aspergillosis model, mice treated with E1210, voriconazole, or caspofungin showed significantly higher survival rates than the control mice (P < 0.05). E1210 was also effective in the treatment of Aspergillus fumigatus pulmonary aspergillosis. In contrast to many antifungals, E1210 was also effective against disseminated fusariosis caused by F. solani. In conclusion, E1210 demonstrated consistent efficacy in murine models of oropharyngeal and disseminated candidiasis, pulmonary aspergillosis, and disseminated fusariosis. These data suggest that further studies to determine E1210's potential for the treatment of disseminated fungal infections are indicated.
Figures

Similar articles
-
The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans.Antimicrob Agents Chemother. 2015 Jan;59(1):690-2. doi: 10.1128/AAC.03944-14. Epub 2014 Oct 20. Antimicrob Agents Chemother. 2015. PMID: 25331706 Free PMC article.
-
In vitro activity of a novel broad-spectrum antifungal, E1210, tested against Aspergillus spp. determined by CLSI and EUCAST broth microdilution methods.Antimicrob Agents Chemother. 2011 Nov;55(11):5155-8. doi: 10.1128/AAC.00570-11. Epub 2011 Aug 15. Antimicrob Agents Chemother. 2011. PMID: 21844312 Free PMC article.
-
Efficacy of FK463, a new lipopeptide antifungal agent, in mouse models of disseminated candidiasis and aspergillosis.Antimicrob Agents Chemother. 2000 Mar;44(3):614-8. doi: 10.1128/AAC.44.3.614-618.2000. Antimicrob Agents Chemother. 2000. PMID: 10681327 Free PMC article.
-
Liposomal amphotericin B: a review of its use as empirical therapy in febrile neutropenia and in the treatment of invasive fungal infections.Drugs. 2009;69(3):361-92. doi: 10.2165/00003495-200969030-00010. Drugs. 2009. PMID: 19275278 Review.
-
[New antifungal agents: voriconazole and caspofungin].Arch Pediatr. 2003 Dec;10 Suppl 5:592s-598s. doi: 10.1016/s0929-693x(03)90043-1. Arch Pediatr. 2003. PMID: 15022787 Review. French.
Cited by
-
Fungal Cell Wall: Emerging Antifungals and Drug Resistance.Front Microbiol. 2019 Nov 21;10:2573. doi: 10.3389/fmicb.2019.02573. eCollection 2019. Front Microbiol. 2019. PMID: 31824443 Free PMC article. Review.
-
APX001 Pharmacokinetic/Pharmacodynamic Target Determination against Aspergillus fumigatus in an In Vivo Model of Invasive Pulmonary Aspergillosis.Antimicrob Agents Chemother. 2019 Mar 27;63(4):e02372-18. doi: 10.1128/AAC.02372-18. Print 2019 Apr. Antimicrob Agents Chemother. 2019. PMID: 30670426 Free PMC article.
-
Antifungal resistance: current trends and future strategies to combat.Infect Drug Resist. 2017 Aug 29;10:249-259. doi: 10.2147/IDR.S124918. eCollection 2017. Infect Drug Resist. 2017. PMID: 28919789 Free PMC article. Review.
-
DectiSomes: Glycan Targeting of Liposomal Drugs Improves the Treatment of Disseminated Candidiasis.Antimicrob Agents Chemother. 2022 Jan 18;66(1):e0146721. doi: 10.1128/AAC.01467-21. Epub 2021 Oct 11. Antimicrob Agents Chemother. 2022. PMID: 34633846 Free PMC article.
-
Rodent Models of Invasive Aspergillosis due to Aspergillus fumigatus: Still a Long Path toward Standardization.Front Microbiol. 2017 May 16;8:841. doi: 10.3389/fmicb.2017.00841. eCollection 2017. Front Microbiol. 2017. PMID: 28559881 Free PMC article. Review.
References
-
- Clinical and Laboratory Standards Institute 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi. Approved standard. CLSI document M38-A2. Clinical and Laboratory Standards Institute, Wayne, PA
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

