Optical measurement of cycle-dependent cell growth
- PMID: 21788503
- PMCID: PMC3156192
- DOI: 10.1073/pnas.1100506108
Optical measurement of cycle-dependent cell growth
Abstract
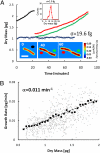
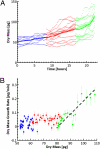
Determining the growth patterns of single cells offers answers to some of the most elusive questions in contemporary cell biology: how cell growth is regulated and how cell size distributions are maintained. For example, a linear growth in time implies that there is no regulation required to maintain homeostasis; an exponential pattern indicates the opposite. Recently, there has been great effort to measure single cells using microelectromechanical systems technology, and several important questions have been explored. However, a unified, easy-to-use methodology to measure the growth rate of individual adherent cells of various sizes has been lacking. Here we demonstrate that a newly developed optical interferometric technique, known as spatial light interference microscopy, can measure the cell dry mass of many individual adherent cells in various conditions, over spatial scales from micrometers to millimeters, temporal scales ranging from seconds to days, and cell types ranging from bacteria to mammalian cells. We found evidence of exponential growth in Escherichia coli, which agrees very well with other recent reports. Perhaps most importantly, combining spatial light interference microscopy with fluorescence imaging provides a unique method for studying cell cycle-dependent growth. Thus, by using a fluorescent reporter for the S phase, we measured single cell growth over each phase of the cell cycle in human osteosarcoma U2OS cells and found that the G2 phase exhibits the highest growth rate, which is mass-dependent and can be approximated by an exponential.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

