Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice
- PMID: 21788509
- PMCID: PMC3156175
- DOI: 10.1073/pnas.1109769108
Transgenic expression of human signal regulatory protein alpha in Rag2-/-gamma(c)-/- mice improves engraftment of human hematopoietic cells in humanized mice
Abstract
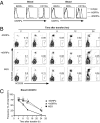
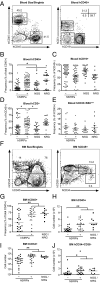
Transplantation of human hematopoietic stem cells into severely immunocompromised newborn mice allows the development of a human hematopoietic and immune system in vivo. NOD/scid/γ(c)(-/-) (NSG) and BALB/c Rag2(-/-)γ(c)(-/-) mice are the most commonly used mouse strains for this purpose and a number of studies have demonstrated the high value of these model systems in areas spanning from basic to translational research. However, limited cross-reactivity of many murine cytokines on human cells and residual host immune function against the xenogeneic grafts results in defective development and maintenance of human cells in vivo. Whereas NSG mice have higher levels of absolute human engraftment than similar mice on a BALB/c background, they have a shorter lifespan and NOD ES cells are unsuitable for the complex genetic engineering that is required to improve human hematopoiesis and immune responses by transgenesis or knockin of human genes. We have generated mice that faithfully express a transgene of human signal regulatory protein alpha (SIRPa), a receptor that negatively regulates phagocytosis, in Rag2(-/-)γ(c)(-/-) mice on a mixed 129/BALB/c background, which can easily be genetically engineered. These mice allow significantly increased engraftment and maintenance of human hematopoietic cells reaching levels comparable to NSG mice. Furthermore, we found improved functionality of the human immune system in these mice. In summary, hSIRPa-transgenic Rag2(-/-)γ(c)(-/-) mice represent a unique mouse strain supporting high levels of human cell engraftment, which can easily be genetically manipulated.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Mestas J, Hughes CC. Of mice and not men: Differences between mouse and human immunology. J Immunol. 2004;172:2731–2738. - PubMed
-
- Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. - PubMed
-
- Manz MG. Human-hemato-lymphoid-system mice: Opportunities and challenges. Immunity. 2007;26:537–541. - PubMed
-
- Traggiai E, et al. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

