Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1)
- PMID: 21788510
- PMCID: PMC3156176
- DOI: 10.1073/pnas.1101664108
Stoichiometric requirements for trapping and gating of Ca2+ release-activated Ca2+ (CRAC) channels by stromal interaction molecule 1 (STIM1)
Abstract
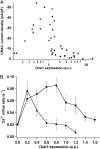
Store-operated Ca(2+) entry depends critically on physical interactions of the endoplasmic reticulum (ER) Ca(2+) sensor stromal interaction molecule 1 (STIM1) and the Ca(2+) release-activated Ca(2+) (CRAC) channel protein Orai1. Recent studies support a diffusion-trap mechanism in which ER Ca(2+) depletion causes STIM1 to accumulate at ER-plasma membrane (PM) junctions, where it binds to Orai1, trapping and activating mobile CRAC channels in the overlying PM. To determine the stoichiometric requirements for CRAC channel trapping and activation, we expressed mCherry-STIM1 and Orai1-GFP at varying ratios in HEK cells and quantified CRAC current (I(CRAC)) activation and the STIM1:Orai1 ratio at ER-PM junctions after store depletion. By competing for a limited amount of STIM1, high levels of Orai1 reduced the junctional STIM1:Orai1 ratio to a lower limit of 0.3-0.6, indicating that binding of one to two STIM1s is sufficient to immobilize the tetrameric CRAC channel at ER-PM junctions. In cells expressing a constant amount of STIM1, CRAC current was a highly nonlinear bell-shaped function of Orai1 expression and the minimum stoichiometry for channel trapping failed to evoke significant activation. Peak current occurred at a ratio of ∼2 STIM1:Orai1, suggesting that maximal CRAC channel activity requires binding of eight STIM1s to each channel. Further increases in Orai1 caused channel activity and fast Ca(2+)-dependent inactivation to decline in parallel. The data are well described by a model in which STIM1 binds to Orai1 with negative cooperativity and channels open with positive cooperativity as a result of stabilization of the open state by STIM1.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


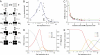
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

