A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits
- PMID: 21788520
- PMCID: PMC3161534
- DOI: 10.1073/pnas.1101767108
A diversity of paracrine signals sustains molecular circadian cycling in suprachiasmatic nucleus circuits
Abstract
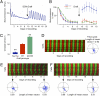
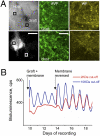
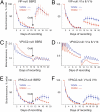
The suprachiasmatic nucleus (SCN) is the principal circadian pacemaker of mammals, coordinating daily rhythms of behavior and metabolism. Circadian timekeeping in SCN neurons revolves around transcriptional/posttranslational feedback loops, in which Period (Per) and Cryptochrome (Cry) genes are negatively regulated by their protein products. Recent studies have revealed, however, that these "core loops" also rely upon cytosolic and circuit-level properties for sustained oscillation. To characterize interneuronal signals responsible for robust pacemaking in SCN cells and circuits, we have developed a unique coculture technique using wild-type (WT) "graft" SCN to drive pacemaking (reported by PER2::LUCIFERASE bioluminescence) in "host" SCN deficient either in elements of neuropeptidergic signaling or in elements of the core feedback loop. We demonstrate that paracrine signaling is sufficient to restore cellular synchrony and amplitude of pacemaking in SCN circuits lacking vasoactive intestinal peptide (VIP). By using grafts with mutant circadian periods we show that pacemaking in the host SCN is specified by the genotype of the graft, confirming graft-derived factors as determinants of the host rhythm. By combining pharmacological with genetic manipulations, we show that a hierarchy of neuropeptidergic signals underpins this paracrine regulation, with a preeminent role for VIP augmented by contributions from arginine vasopressin (AVP) and gastrin-releasing peptide (GRP). Finally, we show that interneuronal signaling is sufficiently powerful to maintain circadian pacemaking in arrhythmic Cry-null SCN, deficient in essential elements of the transcriptional negative feedback loops. Thus, a hierarchy of paracrine neuropeptidergic signals determines cell- and circuit-level circadian pacemaking in the SCN.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Comment in
-
Neuropeptides go the distance for circadian synchrony.Proc Natl Acad Sci U S A. 2011 Aug 23;108(34):13883-4. doi: 10.1073/pnas.1110844108. Epub 2011 Aug 11. Proc Natl Acad Sci U S A. 2011. PMID: 21836054 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

