Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group
- PMID: 21788564
- PMCID: PMC3158598
- DOI: 10.1200/JCO.2010.34.1578
Phase II study of temsirolimus in women with recurrent or metastatic endometrial cancer: a trial of the NCIC Clinical Trials Group
Abstract
Purpose: Phosphatase and tensin homolog (PTEN) is a tumor suppressor gene, and loss of function mutations are common and appear to be important in the pathogenesis of endometrial carcinomas. Loss of PTEN causes deregulated phosphatidylinositol-3 kinase/serine-threonine kinase/mammalian target of rapamycin (PI3K/Akt/mTOR) signaling which may provide neoplastic cells with a selective survival advantage by enhancing angiogenesis, protein translation, and cell cycle progression. Temsirolimus, an ester derivative of rapamycin that inhibits mTOR, was evaluated in this setting.
Patients and methods: Sequential phase II studies evaluated single-agent activity of temsirolimus in women with recurrent or metastatic chemotherapy-naive or chemotherapy-treated endometrial cancer. Temsirolimus 25 mg intravenously was administered weekly in 4-week cycles.
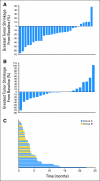
Results: In the chemotherapy-naive group, 33 patients received a median of four cycles (range, one to 23 cycles). Of the 29 patients evaluable for response, four (14%) had an independently confirmed partial response and 20 (69%) had stable disease as best response, with a median duration of 5.1 months (range, 3.7 to 18.4 months) and 9.7 months (range, 2.1 to 14.6 months). Only five patients (18%) had progressive disease. In the chemotherapy-treated group, 27 patients received a median of three cycles (range, one to six cycles). Of the 25 patients evaluable for response, one (4%) had an independently confirmed partial response, and 12 patients (48%) had stable disease, with a median duration of 4.3 months (range, 3.6 to 4.9 months) and 3.7 months (range, 2.4 to 23.2 months). PTEN loss (immunohistochemistry and mutational analysis) and molecular markers of PI3K/Akt/mTOR pathway did not correlate with the clinical outcome.
Conclusion: mTOR inhibition with temsirolimus has encouraging single-agent activity in endometrial cancer which is higher in chemotherapy-naive patients than in chemotherapy-treated patients and is independent of PTEN status. The difference in activity according to prior therapy should be factored into future clinical trial designs.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. - PubMed
-
- Emons G, Heyl W. Hormonal treatment of endometrial cancer. J Cancer Res Clin Oncol. 2000;126:619–623. - PubMed
-
- Lentz SS, Brady MF, Major FJ, et al. High-dose megestrol acetate in advanced or recurrent endometrial carcinoma: A Gynecologic Oncology Group Study. J Clin Oncol. 1996;14:357–361. - PubMed
-
- Podratz KC, O'Brien PC, Malkasian GD, Jr, et al. Effects of progestational agents in treatment of endometrial carcinoma. Obstet Gynecol. 1985;66:106–110. - PubMed
-
- Quinn MA, Cauchi M, Fortune D. Endometrial carcinoma: Steroid receptors and response to medroxyprogesterone acetate. Gynecol Oncol. 1985;21:314–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

