Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle
- PMID: 21788611
- PMCID: PMC3149434
- DOI: 10.1085/jgp.201010592
Measurement of RyR permeability reveals a role of calsequestrin in termination of SR Ca(2+) release in skeletal muscle
Abstract
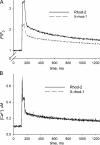
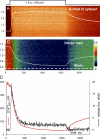
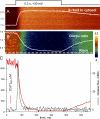
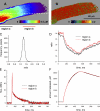
The mechanisms that terminate Ca(2+) release from the sarcoplasmic reticulum are not fully understood. D4cpv-Casq1 (Sztretye et al. 2011. J. Gen. Physiol. doi:10.1085/jgp.201010591) was used in mouse skeletal muscle cells under voltage clamp to measure free Ca(2+) concentration inside the sarcoplasmic reticulum (SR), [Ca(2+)](SR), simultaneously with that in the cytosol, [Ca(2+)](c), during the response to long-lasting depolarization of the plasma membrane. The ratio of Ca(2+) release flux (derived from [Ca(2+)](c)(t)) over the gradient that drives it (essentially equal to [Ca(2+)](SR)) provided directly, for the first time, a dynamic measure of the permeability to Ca(2+) of the releasing SR membrane. During maximal depolarization, flux rapidly rises to a peak and then decays. Before 0.5 s, [Ca(2+)](SR) stabilized at ∼35% of its resting level; depletion was therefore incomplete. By 0.4 s of depolarization, the measured permeability decayed to ∼10% of maximum, indicating ryanodine receptor channel closure. Inactivation of the t tubule voltage sensor was immeasurably small by this time and thus not a significant factor in channel closure. In cells of mice null for Casq1, permeability did not decrease in the same way, indicating that calsequestrin (Casq) is essential in the mechanism of channel closure and termination of Ca(2+) release. The absence of this mechanism explains why the total amount of calcium releasable by depolarization is not greatly reduced in Casq-null muscle (Royer et al. 2010. J. Gen. Physiol. doi:10.1085/jgp.201010454). When the fast buffer BAPTA was introduced in the cytosol, release flux became more intense, and the SR emptied earlier. The consequent reduction in permeability accelerated as well, reaching comparable decay at earlier times but comparable levels of depletion. This observation indicates that [Ca(2+)](SR), sensed by Casq and transmitted to the channels presumably via connecting proteins, is determinant to cause the closure that terminates Ca(2+) release.
Figures





Similar articles
-
Mice null for calsequestrin 1 exhibit deficits in functional performance and sarcoplasmic reticulum calcium handling.PLoS One. 2011;6(12):e27036. doi: 10.1371/journal.pone.0027036. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164205 Free PMC article.
-
D4cpv-calsequestrin: a sensitive ratiometric biosensor accurately targeted to the calcium store of skeletal muscle.J Gen Physiol. 2011 Aug;138(2):211-29. doi: 10.1085/jgp.201010591. J Gen Physiol. 2011. PMID: 21788610 Free PMC article.
-
Paradoxical buffering of calcium by calsequestrin demonstrated for the calcium store of skeletal muscle.J Gen Physiol. 2010 Sep;136(3):325-38. doi: 10.1085/jgp.201010454. Epub 2010 Aug 16. J Gen Physiol. 2010. PMID: 20713548 Free PMC article.
-
Calsequestrin: a well-known but curious protein in skeletal muscle.Exp Mol Med. 2020 Dec;52(12):1908-1925. doi: 10.1038/s12276-020-00535-1. Epub 2020 Dec 7. Exp Mol Med. 2020. PMID: 33288873 Free PMC article. Review.
-
Calsequestrin, a key protein in striated muscle health and disease.J Muscle Res Cell Motil. 2021 Jun;42(2):267-279. doi: 10.1007/s10974-020-09583-6. Epub 2020 Jun 2. J Muscle Res Cell Motil. 2021. PMID: 32488451 Review.
Cited by
-
Synthetic localized calcium transients directly probe signalling mechanisms in skeletal muscle.J Physiol. 2012 Mar 15;590(6):1389-411. doi: 10.1113/jphysiol.2011.225854. Epub 2012 Feb 6. J Physiol. 2012. PMID: 22310315 Free PMC article.
-
Characterization of Two Human Skeletal Calsequestrin Mutants Implicated in Malignant Hyperthermia and Vacuolar Aggregate Myopathy.J Biol Chem. 2015 Nov 27;290(48):28665-74. doi: 10.1074/jbc.M115.686261. Epub 2015 Sep 28. J Biol Chem. 2015. PMID: 26416891 Free PMC article.
-
Altered Ca2+ concentration, permeability and buffering in the myofibre Ca2+ store of a mouse model of malignant hyperthermia.J Physiol. 2013 Sep 15;591(18):4439-57. doi: 10.1113/jphysiol.2013.259572. Epub 2013 Jun 24. J Physiol. 2013. PMID: 23798496 Free PMC article.
-
Calcium buffering properties of sarcoplasmic reticulum and calcium-induced Ca(2+) release during the quasi-steady level of release in twitch fibers from frog skeletal muscle.J Gen Physiol. 2012 Oct;140(4):403-19. doi: 10.1085/jgp.201110730. J Gen Physiol. 2012. PMID: 23008434 Free PMC article.
-
Mice null for calsequestrin 1 exhibit deficits in functional performance and sarcoplasmic reticulum calcium handling.PLoS One. 2011;6(12):e27036. doi: 10.1371/journal.pone.0027036. Epub 2011 Dec 2. PLoS One. 2011. PMID: 22164205 Free PMC article.
References
-
- Canato M., Scorzeto M., Giacomello M., Protasi F., Reggiani C., Stienen G.J. 2010. Massive alterations of sarcoplasmic reticulum free calcium in skeletal muscle fibers lacking calsequestrin revealed by a genetically encoded probe. Proc. Natl. Acad. Sci. USA. 107:22326–22331 10.1073/pnas.1009168108 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

