P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis
- PMID: 21788941
- PMCID: PMC3909679
- DOI: 10.1038/labinvest.2011.113
P-glycoprotein induction by breast milk attenuates intestinal inflammation in experimental necrotizing enterocolitis
Abstract
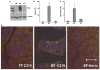
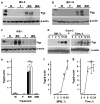
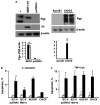
P-glycoprotein (Pgp), a product of the multi-drug resistance gene MDR1a, is a broad specificity efflux ATP cassette transmembrane transporter that is predominantly expressed in epithelial tissues. Because mdr1a(-/-) mice tend to develop spontaneous colitis in bacteria-dependent manner, Pgp is believed to have a role in protection of the intestinal epithelium from luminal bacteria. Here we demonstrate that levels of Pgp in the small intestine of newborn rodents dramatically increase during breastfeeding, but not during formula feeding (FF). In rats and mice, levels of intestinal Pgp peak on days 3-7 and 1-5 of breastfeeding, respectively. The mdr1a(-/-) neonatal mice subjected to FF, hypoxia, and hypothermia have significantly higher incidence and pathology, as well as significantly earlier onset of necrotizing enterocolitis (NEC) than congenic wild type mice. Breast-fed mdr1a(-/-) neonatal mice are also more susceptible to intestinal damage caused by the opportunistic pathogen Cronobacter sakazakii that has been associated with hospital outbreaks of NEC. Breast milk, but not formula, induces Pgp expression in enterocyte cell lines in a dose- and time-dependent manner. High levels of ectopically expressed Pgp protect epithelial cells in vitro from apoptosis induced by C. sakazakii. Taken together, these results show that breast milk-induced expression of Pgp may have a role in the protection of the neonatal intestinal epithelium from injury associated with nascent bacterial colonization.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Guthrie SO, Gordon PV, Thomas V, et al. Necrotizing enterocolitis among neonates in the United States. J Perinatol. 2003;23:278–285. - PubMed
-
- Hammerman C, Bin-Nun A, Kaplan M. Germ warfare: probiotics in defense of the premature gut. Clin Perinatol. 2004;31:489–500. - PubMed
-
- Morowitz MJ, Poroyko V, Caplan M, et al. Redefining the role of intestinal microbes in the pathogenesis of necrotizing enterocolitis. Pediatrics. 125:777–785. - PubMed
-
- Patole S. Prevention and treatment of necrotising enterocolitis in preterm neonates. Early Hum Dev. 2007;83:635–642. - PubMed
-
- Hall NJ, Eaton S, Peters MJ, et al. Mild controlled hypothermia in preterm neonates with advanced necrotizing enterocolitis. Pediatrics. 2010;125:e300–e308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

