IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease
- PMID: 21789181
- PMCID: PMC3138740
- DOI: 10.1371/journal.pone.0021799
IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease
Abstract
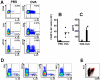
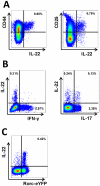
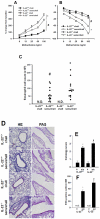
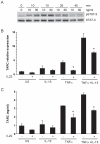
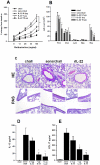
Interleukin (IL)-22 is an effector cytokine, which acts primarily on epithelial cells in the skin, gut, liver and lung. Both pro- and anti-inflammatory properties have been reported for IL-22 depending on the tissue and disease model. In a murine model of allergic airway inflammation, we found that IL-22 is predominantly produced by innate lymphoid cells in the inflamed lungs, rather than TH cells. To determine the impact of IL-22 on airway inflammation, we used allergen-sensitized IL-22-deficient mice and found that they suffer from significantly higher airway hyperreactivity upon airway challenge. IL-22-deficiency led to increased eosinophil infiltration lymphocyte invasion and production of CCL17 (TARC), IL-5 and IL-13 in the lung. Mice treated with IL-22 before antigen challenge displayed reduced expression of CCL17 and IL-13 and significant amelioration of airway constriction and inflammation. We conclude that innate IL-22 limits airway inflammation, tissue damage and clinical decline in allergic lung disease.
Conflict of interest statement
Figures

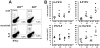
References
-
- Dumoutier L, Louahed J, Renauld JC. Cloning and characterization of IL-10-related T cell-derived inducible factor (IL-TIF), a novel cytokine structurally related to IL-10 and inducible by IL-9. J Immunol. 2000;164:1814–1819. - PubMed
-
- Kotenko SV, Izotova LS, Mirochnitchenko OV, Esterova E, Dickensheets H, et al. Identification of the functional interleukin-22 (IL-22) receptor complex: the IL-10R2 chain (IL-10Rbeta) is a common chain of both the IL-10 and IL-22 (IL-10-related T cell-derived inducible factor, IL-TIF) receptor complexes. J Biol Chem. 2001;276:2725–2732. - PubMed
-
- Xie MH, Aggarwal S, Ho WH, Foster J, Zhang Z, et al. Interleukin (IL)-22, a novel human cytokine that signals through the interferon receptor-related proteins CRF2-4 and IL-22R. J Biol Chem. 2000;275:31335–31339. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

