A novel manganese efflux system, YebN, is required for virulence by Xanthomonas oryzae pv. oryzae
- PMID: 21789199
- PMCID: PMC3136493
- DOI: 10.1371/journal.pone.0021983
A novel manganese efflux system, YebN, is required for virulence by Xanthomonas oryzae pv. oryzae
Abstract
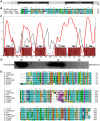
Manganese ions (Mn(2+)) play a crucial role in virulence and protection against oxidative stress in bacterial pathogens. Such pathogens appear to have evolved complex mechanisms for regulating Mn(2+) uptake and efflux. Despite numerous studies on Mn(2+) uptake, however, only one efflux system has been identified to date. Here, we report on a novel Mn(2+) export system, YebN, in Xanthomonas oryzae pv. oryzae (Xoo), the causative agent of bacterial leaf blight. Compared with wild-type PXO99, the yebN mutant was highly sensitive to Mn(2+) and accumulated high concentrations of intracellular manganese. In addition, we found that expression of yebN was positively regulated by Mn(2+) and the Mn(2+)-dependent transcription regulator, MntR. Interestingly, the yebN mutant was more tolerant to methyl viologen and H(2)O(2) in low Mn(2+) medium than PXO99, but more sensitive in high Mn(2+) medium, implying that YebN plays an important role in Mn(2+) homoeostasis and detoxification of reactive oxygen species (ROS). Notably, deletion of yebN rendered Xoo sensitive to hypo-osmotic shock, suggesting that YebN may protect against such stress. That mutation of yebN substantially reduced the Xoo growth rate and lesion formation in rice implies that YebN could be involved in Xoo fitness in host. Although YebN has two DUF204 domains, it lacks homology to any known metal transporter. Hence, this is the first report of a novel metal export system that plays essential roles in hypo-osmotic and oxidative stress, and virulence. Our results lay the foundations for elucidating the complex and fascinating relationship between metal homeostasis and host-pathogen interactions.
Conflict of interest statement
Figures






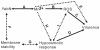
References
-
- Papp-Wallace KM, Maguire ME. Manganese Transport and the Role of Manganese in Virulence. Annu Rev Microbiol. 2006;60:187–209. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

