Histone deacetylase inhibition enhances self renewal and cardioprotection by human cord blood-derived CD34 cells
- PMID: 21789227
- PMCID: PMC3138768
- DOI: 10.1371/journal.pone.0022158
Histone deacetylase inhibition enhances self renewal and cardioprotection by human cord blood-derived CD34 cells
Abstract
Background: Use of peripheral blood- or bone marrow-derived progenitors for ischemic heart repair is a feasible option to induce neo-vascularization in ischemic tissues. These cells, named Endothelial Progenitors Cells (EPCs), have been extensively characterized phenotypically and functionally. The clinical efficacy of cardiac repair by EPCs cells remains, however, limited, due to cell autonomous defects as a consequence of risk factors. The devise of "enhancement" strategies has been therefore sought to improve repair ability of these cells and increase the clinical benefit.
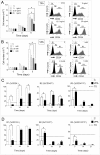
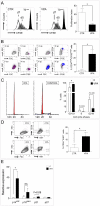
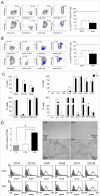
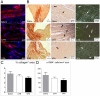
Principal findings: Pharmacologic inhibition of histone deacetylases (HDACs) is known to enhance hematopoietic stem cells engraftment by improvement of self renewal and inhibition of differentiation in the presence of mitogenic stimuli in vitro. In the present study cord blood-derived CD34(+) were pre-conditioned with the HDAC inhibitor Valproic Acid. This treatment affected stem cell growth and gene expression, and improved ischemic myocardium protection in an immunodeficient mouse model of myocardial infarction.
Conclusions: Our results show that HDAC blockade leads to phenotype changes in CD34(+) cells with enhanced self renewal and cardioprotection.
Conflict of interest statement
Figures




Similar articles
-
Histone deacetylase (HDAC) inhibitors down-regulate endothelial lineage commitment of umbilical cord blood derived endothelial progenitor cells.Int J Mol Sci. 2012 Nov 15;13(11):15074-85. doi: 10.3390/ijms131115074. Int J Mol Sci. 2012. PMID: 23203112 Free PMC article.
-
Histone deacetylase 3 modulates the expansion of human hematopoietic stem cells.Stem Cells Dev. 2012 Sep 20;21(14):2581-91. doi: 10.1089/scd.2011.0698. Epub 2012 May 8. Stem Cells Dev. 2012. PMID: 22455388
-
Inhibitors of histone deacetylases promote hematopoietic stem cell self-renewal.Cytotherapy. 2004;6(4):328-36. doi: 10.1080/14653240410004899. Cytotherapy. 2004. PMID: 16146885
-
Expansion and preservation of the functional activity of adult hematopoietic stem cells cultured ex vivo with a histone deacetylase inhibitor.Stem Cells Transl Med. 2020 Apr;9(4):531-542. doi: 10.1002/sctm.19-0199. Epub 2020 Jan 17. Stem Cells Transl Med. 2020. PMID: 31950644 Free PMC article.
-
CD34-positive stem cells: in the treatment of heart and vascular disease in human beings.Tex Heart Inst J. 2011;38(5):474-85. Tex Heart Inst J. 2011. PMID: 22163120 Free PMC article. Review.
Cited by
-
Epigenetic programming and risk: the birthplace of cardiovascular disease?Stem Cell Rev Rep. 2013 Jun;9(3):241-53. doi: 10.1007/s12015-012-9398-z. Stem Cell Rev Rep. 2013. PMID: 22773406 Review.
-
Expression of dual nucleotides/cysteinyl-leukotrienes receptor GPR17 in early trafficking of cardiac stromal cells after myocardial infarction.J Cell Mol Med. 2014 Sep;18(9):1785-96. doi: 10.1111/jcmm.12305. Epub 2014 Jun 7. J Cell Mol Med. 2014. PMID: 24909956 Free PMC article.
-
Transplantation of Epigenetically Modified Adult Cardiac c-Kit+ Cells Retards Remodeling and Improves Cardiac Function in Ischemic Heart Failure Model.Stem Cells Transl Med. 2015 Sep;4(9):1086-96. doi: 10.5966/sctm.2014-0290. Epub 2015 Aug 3. Stem Cells Transl Med. 2015. PMID: 26240433 Free PMC article.
-
Histone Deacetylase Inhibitors as Anticancer Drugs.Int J Mol Sci. 2017 Jul 1;18(7):1414. doi: 10.3390/ijms18071414. Int J Mol Sci. 2017. PMID: 28671573 Free PMC article. Review.
-
Histone deacetylase (HDAC) inhibitors down-regulate endothelial lineage commitment of umbilical cord blood derived endothelial progenitor cells.Int J Mol Sci. 2012 Nov 15;13(11):15074-85. doi: 10.3390/ijms131115074. Int J Mol Sci. 2012. PMID: 23203112 Free PMC article.
References
-
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, et al. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. - PubMed
-
- Rossig L, Urbich C, Dimmeler S. Endothelial progenitor cells at work: not mature yet, but already stress-resistant. Arterioscler Thromb Vasc Biol. 2004;24:1977–1979. - PubMed
-
- Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. - PubMed
-
- Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4(Suppl 1):S110–113. - PubMed
-
- Burba I, Devanna P, Pesce M. When cells become a drug. Endothelial progenitor cells for cardiovascular therapy: aims and reality. Recent Pat Cardiovasc Drug Discov. 2010;5:1–10. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

