Herpes simplex virus type 1 penetrates the basement membrane in human nasal respiratory mucosa
- PMID: 21789229
- PMCID: PMC3137608
- DOI: 10.1371/journal.pone.0022160
Herpes simplex virus type 1 penetrates the basement membrane in human nasal respiratory mucosa
Abstract
Background: Herpes simplex virus infections are highly prevalent in humans. However, the current therapeutics suffer important drawbacks such as limited results in neonates, increasing occurrence of resistance and impeded treatment of stromal infections. Remarkably, interactions of herpesviruses with human mucosa, the locus of infection, remain poorly understood and the underlying mechanisms in stromal infection remain controversial.
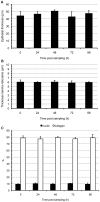
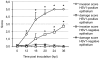
Methodology/principal findings: A human model consisting of nasal respiratory mucosa explants was characterised. Viability and integrity were examined during 96 h of cultivation. HSV1-mucosa interactions were analysed. In particular, we investigated whether HSV1 is able to reach the stroma. Explant viability and integrity remained preserved. HSV1 induced rounding up and loosening of epithelial cells with very few apoptotic and necrotic cells observed. Following 16-24 h of infection, HSV1 penetrated the basement membrane and replicated in the underlying lamina propria.
Conclusions/significance: This human explant model can be used to study virus-mucosa interactions and viral mucosal invasion mechanisms. Using this model, our results provide a novel insight into the HSV1 stromal invasion mechanism and for the first time directly demonstrate that HSV1 can penetrate the basement membrane.
Conflict of interest statement
Figures



References
-
- Marshall DS, Linfert DR, Draghi A, McCarter YS, Tsongalis GJ. Identification of herpes simplex virus genital infection: comparison of a multiplex PCR assay and traditional viral isolation techniques. Mod Pathol. 2001;14:152–156. - PubMed
-
- Whitley RJ. Herpes simplex viruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martint MA, et al., editors. Fields virology. Philadelphia: Lippincott, Williams, Wilkins; 2001. pp. 2461–2509.
-
- Fleming DT, McQuillan GM, Johnson RE, Nahmias AJ, Aral SO, et al. Herpes simplex virus type 2 in the United States, 1976 to 1994. N Engl J Med. 1997;337:1105–111. - PubMed
-
- Mitchell BM, Bloom DC, Cohrs RJ, Gilden DH, Kennedy PG. Herpes simplex virus-1 and varicella-zoster virus latency in ganglia. J Neurovirol. 2003;9:194–204. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

