Production of recombinant human DNA polymerase delta in a Bombyx mori bioreactor
- PMID: 21789240
- PMCID: PMC3137619
- DOI: 10.1371/journal.pone.0022224
Production of recombinant human DNA polymerase delta in a Bombyx mori bioreactor
Abstract
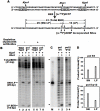
Eukaryotic DNA polymerase δ (pol δ) plays a crucial role in chromosomal DNA replication and various DNA repair processes. It is thought to consist of p125, p66 (p68), p50 and p12 subunits. However, rigorous isolation of mammalian pol δ from natural sources has usually yielded two-subunit preparations containing only p125 and p50 polypeptides. While recombinant pol δ isolated from infected insect cells have some problems of consistency in the quality of the preparations, and the yields are much lower. To address these deficiencies, we have constructed recombinant BmNPV baculoviruses using MultiBac system. This method makes the generation of recombinant forms of pol δ containing mutations in any one of the subunits or combinations thereof extremely facile. From about 350 infected larvae, we obtained as much as 4 mg of pol δ four-subunit complex. Highly purified enzyme behaved like the one of native form by rigorous characterization and comparison of its activities on poly(dA)/oligo(dT) template-primer and singly primed M13 DNA, and its homogeneity on FPLC gel filtration. In vitro base excision repair (BER) assays showed that pol δ plays a significant role in uracil-intiated BER and is more likely to mediate LP BER, while the trimer lacking p12 is more likely to mediate SN BER. It seems likely that loss of p12 modulates the rate of SN BER and LP BER during the repair process. Thus, this work provides a simple, fast, reliable and economic way for the large-scale production of human DNA polymerase δ with a high activity and purity, setting up a new platform for our further research on the biochemical properties of pol δ, its regulation and the integration of its functions, and how alterations in pol δ function could contribute to the etiology of human cancer or other diseases that can result from loss of genomic stability.
Conflict of interest statement
Figures






Similar articles
-
Reconstitution of human DNA polymerase delta using recombinant baculoviruses: the p12 subunit potentiates DNA polymerizing activity of the four-subunit enzyme.J Biol Chem. 2002 Feb 8;277(6):3894-901. doi: 10.1074/jbc.M109684200. Epub 2001 Nov 15. J Biol Chem. 2002. PMID: 11711545
-
Characterization of human DNA polymerase delta and its subassemblies reconstituted by expression in the MultiBac system.PLoS One. 2012;7(6):e39156. doi: 10.1371/journal.pone.0039156. Epub 2012 Jun 18. PLoS One. 2012. PMID: 22723953 Free PMC article.
-
Characterization of the human DNA polymerase delta catalytic subunit expressed by a recombinant baculovirus.Nagoya J Med Sci. 2000 Nov;63(3-4):99-113. Nagoya J Med Sci. 2000. PMID: 11201990
-
Multiple Forms of Human DNA Polymerase Delta Sub-Assembling in Cellular DNA Transactions.Curr Protein Pept Sci. 2016;17(8):746-755. doi: 10.2174/1389203717666160226145006. Curr Protein Pept Sci. 2016. PMID: 26916162 Review.
-
The p12 Subunit Choreographs the Regulation and Functions of Two Forms of DNA Polymerase δ in Mammalian Cells.Genes (Basel). 2025 Feb 3;16(2):188. doi: 10.3390/genes16020188. Genes (Basel). 2025. PMID: 40004517 Free PMC article. Review.
Cited by
-
P50, the small subunit of DNA polymerase delta, is required for mediation of the interaction of polymerase delta subassemblies with PCNA.PLoS One. 2011;6(11):e27092. doi: 10.1371/journal.pone.0027092. Epub 2011 Nov 2. PLoS One. 2011. PMID: 22073260 Free PMC article.
-
Silkworm: A Promising Model Organism in Life Science.J Insect Sci. 2017 Sep 1;17(5):97. doi: 10.1093/jisesa/iex064. J Insect Sci. 2017. PMID: 29117372 Free PMC article. Review.
-
DNA polymerase delta in DNA replication and genome maintenance.Environ Mol Mutagen. 2012 Dec;53(9):666-82. doi: 10.1002/em.21745. Epub 2012 Oct 13. Environ Mol Mutagen. 2012. PMID: 23065663 Free PMC article. Review.
-
Human DNA polymerase delta is a pentameric holoenzyme with a dimeric p12 subunit.Life Sci Alliance. 2019 Mar 18;2(2):e201900323. doi: 10.26508/lsa.201900323. Print 2019 Apr. Life Sci Alliance. 2019. PMID: 30885984 Free PMC article.
-
Expression analysis of Bombyx mori bidensovirus structural proteins and assembly of virus-like particles in insect cells.Curr Microbiol. 2014 Oct;69(4):567-73. doi: 10.1007/s00284-014-0613-9. Epub 2014 Jun 12. Curr Microbiol. 2014. PMID: 24916668
References
-
- Johnson A, O'Donnell M. Cellular DNA replicases: components and dynamics at the replication fork. Annu Rev Biochem. 2005;74:283–315. - PubMed
-
- Takeda DY, Dutta A. DNA replication and progression through S phase. Oncogene. 2005;24:2827–2843. - PubMed
-
- Garg P, Burgers PM. DNA polymerases that propagate the eukaryotic DNA replication fork. Crit Rev Biochem Mol Biol. 2005;40:115–128. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

