Energy metabolism in H460 lung cancer cells: effects of histone deacetylase inhibitors
- PMID: 21789245
- PMCID: PMC3138778
- DOI: 10.1371/journal.pone.0022264
Energy metabolism in H460 lung cancer cells: effects of histone deacetylase inhibitors
Abstract
Background: Tumor cells are characterized by accelerated growth usually accompanied by up-regulated pathways that ultimately increase the rate of ATP production. These cells can suffer metabolic reprogramming, resulting in distinct bioenergetic phenotypes, generally enhancing glycolysis channeled to lactate production. In the present work we showed metabolic reprogramming by means of inhibitors of histone deacetylase (HDACis), sodium butyrate and trichostatin. This treatment was able to shift energy metabolism by activating mitochondrial systems such as the respiratory chain and oxidative phosphorylation that were largely repressed in the untreated controls.
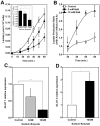
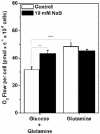
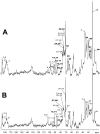
Methodology/principal findings: Various cellular and biochemical parameters were evaluated in lung cancer H460 cells treated with the histone deacetylase inhibitors (HDACis), sodium butyrate (NaB) and trichostatin A (TSA). NaB and TSA reduced glycolytic flux, assayed by lactate release by H460 cells in a concentration dependent manner. NaB inhibited the expression of glucose transporter type 1 (GLUT 1), but substantially increased mitochondria bound hexokinase (HK) activity. NaB induced increase in HK activity was associated to isoform HK I and was accompanied by 1.5 fold increase in HK I mRNA expression and cognate protein biosynthesis. Lactate dehydrogenase (LDH) and pyruvate kinase (PYK) activities were unchanged by HDACis suggesting that the increase in the HK activity was not coupled to glycolytic flux. High resolution respirometry of H460 cells revealed NaB-dependent increased rates of oxygen consumption coupled to ATP synthesis. Metabolomic analysis showed that NaB altered the glycolytic metabolite profile of intact H460 cells. Concomitantly we detected an activation of the pentose phosphate pathway (PPP). The high O(2) consumption in NaB-treated cells was shown to be unrelated to mitochondrial biogenesis since citrate synthase (CS) activity and the amount of mitochondrial DNA remained unchanged.
Conclusion: NaB and TSA induced an increase in mitochondrial function and oxidative metabolism in H460 lung tumor cells concomitant with a less proliferative cellular phenotype.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

