Pre & postsynaptic tuning of action potential timing by spontaneous GABAergic activity
- PMID: 21789249
- PMCID: PMC3137631
- DOI: 10.1371/journal.pone.0022322
Pre & postsynaptic tuning of action potential timing by spontaneous GABAergic activity
Abstract
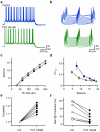
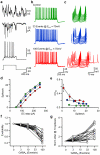
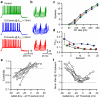
Frequency and timing of action potential discharge are key elements for coding and transfer of information between neurons. The nature and location of the synaptic contacts, the biophysical parameters of the receptor-operated channels and their kinetics of activation are major determinants of the firing behaviour of each individual neuron. Ultimately the intrinsic excitability of each neuron determines the input-output function. Here we evaluate the influence of spontaneous GABAergic synaptic activity on the timing of action potentials in Layer 2/3 pyramidal neurones in acute brain slices from the somatosensory cortex of young rats. Somatic dynamic current injection to mimic synaptic input events was employed, together with a simple computational model that reproduce subthreshold membrane properties. Besides the well-documented control of neuronal excitability, spontaneous background GABAergic activity has a major detrimental effect on spike timing. In fact, GABA(A) receptors tune the relationship between the excitability and fidelity of pyramidal neurons via a postsynaptic (the reversal potential for GABA(A) activity) and a presynaptic (the frequency of spontaneous activity) mechanism. GABAergic activity can decrease or increase the excitability of pyramidal neurones, depending on the difference between the reversal potential for GABA(A) receptors and the threshold for action potential. In contrast, spike time jitter can only be increased proportionally to the difference between these two membrane potentials. Changes in excitability by background GABAergic activity can therefore only be associated with deterioration of the reliability of spike timing.
Conflict of interest statement
Figures




Similar articles
-
Removal of GABAergic inhibition alters subthreshold input in neurons in forepaw barrel subfield (FBS) in rat first somatosensory cortex (SI) after digit stimulation.Exp Brain Res. 2002 Aug;145(4):411-28. doi: 10.1007/s00221-002-1124-7. Epub 2002 Jul 3. Exp Brain Res. 2002. PMID: 12172653
-
Multiple effects of dopamine on layer V pyramidal cell excitability in rat prefrontal cortex.J Neurophysiol. 2001 Aug;86(2):586-95. doi: 10.1152/jn.2001.86.2.586. J Neurophysiol. 2001. PMID: 11495934
-
Distinct types of ionic modulation of GABA actions in pyramidal cells and interneurons during electrical induction of hippocampal seizure-like network activity.Eur J Neurosci. 2007 May;25(9):2713-25. doi: 10.1111/j.1460-9568.2007.05543.x. Epub 2007 Apr 25. Eur J Neurosci. 2007. PMID: 17459104
-
Intracellular correlate of EPSP-spike potentiation in CA1 pyramidal neurons is controlled by GABAergic modulation.Hippocampus. 2003;13(7):801-5. doi: 10.1002/hipo.10129. Hippocampus. 2003. PMID: 14620875
-
Characterisation of rat superficial superior colliculus neurones: firing properties and sensitivity to GABA.Neuroscience. 2002;110(1):93-104. doi: 10.1016/s0306-4522(01)00558-9. Neuroscience. 2002. PMID: 11882375
Cited by
-
Loss of GABAB -mediated interhemispheric synaptic inhibition in stroke periphery.J Physiol. 2018 May 15;596(10):1949-1964. doi: 10.1113/JP275690. Epub 2018 Apr 17. J Physiol. 2018. PMID: 29508394 Free PMC article.
-
Repeated cocaine exposure increases fast-spiking interneuron excitability in the rat medial prefrontal cortex.J Neurophysiol. 2013 Jun;109(11):2781-92. doi: 10.1152/jn.00596.2012. Epub 2013 Mar 13. J Neurophysiol. 2013. PMID: 23486201 Free PMC article.
-
A computational analysis of signal fidelity in the rostral nucleus of the solitary tract.J Neurophysiol. 2018 Mar 1;119(3):771-785. doi: 10.1152/jn.00624.2017. Epub 2017 Nov 1. J Neurophysiol. 2018. PMID: 29093172 Free PMC article.
-
Increased BDNF protein expression after ischemic or PKC epsilon preconditioning promotes electrophysiologic changes that lead to neuroprotection.J Cereb Blood Flow Metab. 2015 Jan;35(1):121-30. doi: 10.1038/jcbfm.2014.185. Epub 2014 Nov 5. J Cereb Blood Flow Metab. 2015. PMID: 25370861 Free PMC article.
-
Self-tuning of inhibition by endocannabinoids shapes spike-time precision in CA1 pyramidal neurons.J Neurophysiol. 2013 Oct;110(8):1930-44. doi: 10.1152/jn.00099.2013. Epub 2013 Jul 31. J Neurophysiol. 2013. PMID: 23904493 Free PMC article.
References
-
- Schneidman E, Freedman B, Segev I. Ion channel stochasticity may be critical in determining the reliability and precision of spike timing. Neural Comput. 1998;10:1679–1703. - PubMed
-
- Arieli A, Sterkin A, Grinvald A, Aertsen A. Dynamics of ongoing activity: Explanation of the large variability in evoked cortical responses. Science. 1996;273:1868–1871. - PubMed
-
- Tsodyks M, Kenet T, Grinvald A, Arieli A. Linking spontaneous activity of single cortical neurons and the underlying functional architecture. Science. 1999;286:1943–1946. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

