Alloimmunization to transfused HOD red blood cells is not increased in mice with sickle cell disease
- PMID: 21790627
- PMCID: PMC3218203
- DOI: 10.1111/j.1537-2995.2011.03255.x
Alloimmunization to transfused HOD red blood cells is not increased in mice with sickle cell disease
Abstract
Background: Increased rates of red blood cell (RBC) alloimmunization in patients with sickle cell disease may be due to transfusion frequency, genetic predisposition, or immune dysregulation. To test the hypothesis that sickle cell pathophysiology influences RBC alloimmunization, we utilized two transgenic mouse models of sickle cell disease.
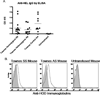
Study design and methods: Transgenic sickle mice, which express human α and β(S) globin, were transfused with fresh or 14-day-stored RBCs containing the HOD (hen egg lysozyme, ovalbumin, and human Duffy(b) ) antigen; some recipients were inflamed with poly(I : C) before transfusion. Anti-HOD alloantibody responses were subsequently measured by enzyme-linked immunosorbent assay and flow crossmatch; a cohort of recipients had posttransfusion serum cytokines measured by bead array.
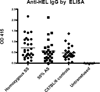
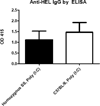
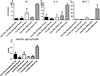
Results: Both Berkeley and Townes homozygous (SS) and heterozygous (AS) mice had similar rates and magnitude of anti-HOD RBC alloimmunization after fresh HOD RBC transfusion compared with control animals; under no tested condition did homozygous SS recipients make higher levels of alloantibodies than control animals. Unexpectedly, homozygous SS recipients had blunted cytokine responses and lower levels of anti-HOD alloantibodies after transfusion of 14-day stored RBCs, compared with control animals.
Conclusions: In sum, homozygous β(S) expression and the ensuing disease state are not alone sufficient to enhance RBC alloimmunization to transfused HOD RBCs in two distinct humanized murine models of sickle cell disease under the conditions examined. These data suggest that other factors may contribute to the high rates of RBC alloimmunization observed in humans with sickle cell disease.
© 2012 American Association of Blood Banks.
Conflict of interest statement
Figures


Comment in
-
Red blood cell alloimmunization in sickle cell anemia: more questions than answers?Transfusion. 2012 Feb;52(2):214-6. doi: 10.1111/j.1537-2995.2011.03502.x. Transfusion. 2012. PMID: 22239207 No abstract available.
References
-
- Aygun B, Padmanabhan S, Paley C, Chandrasekaran V. Clinical significance of RBC alloantibodies and autoantibodies in sickle cell patients who received transfusions. Transfusion. 2002;42(1):37–43. - PubMed
-
- Rosse WF, Gallagher D, Kinney TR, et al. Transfusion and alloimmunization in sickle cell disease. The Cooperative Study of Sickle Cell Disease. Blood. 1990;76(7):1431–1437. - PubMed
-
- Vichinsky EP, Earles A, Johnson RA, Hoag MS, Williams A, Lubin B. Alloimmunization in sickle cell anemia and transfusion of racially unmatched blood.[see comment] New England Journal of Medicine. 1990;322(23):1617–1621. - PubMed
-
- Heddle NM, Soutar RL, O'Hoski PL, et al. A prospective study to determine the frequency and clinical significance of alloimmunization post-transfusion.[see comment] British Journal of Haematology. 1995;91(4):1000–1005. - PubMed
-
- Hoeltge GA, Domen RE, Rybicki LA, Schaffer PA. Multiple red cell transfusions and alloimmunization. Experience with 6996 antibodies detected in a total of 159,262 patients from 1985 to 1993. Archives of Pathology & Laboratory Medicine. 1995;119(1):42–45. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

