Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats
- PMID: 21790671
- PMCID: PMC3204341
- DOI: 10.1111/j.1530-0277.2011.01577.x
Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats
Abstract
Background: Binge drinking after chronic ethanol consumption is one of the important factors contributing to the progression of steatosis to steatohepatitis. The molecular mechanisms of this effect remain poorly understood. We have therefore examined in rats the effect of single and repeat ethanol binge superimposed on chronic ethanol intake on liver injury, activation of mitogen-activated protein kinases (MAPKs), and gene expression.
Methods: Rats were chronically treated with ethanol in liquid diet for 4 weeks followed by single ethanol binge (5 gm/kg body weight) or 3 similar repeated doses of ethanol. Serum alcohol and alanine amino transferase (ALT) levels were determined by enzymatic methods. Steatosis was assessed by histology and hepatic triglycerides. Activation of MAPK, 90S ribosomal kinase (RSK), and caspase 3 were evaluated by Western blot. Levels of mRNA for tumor necrosis factor alpha (TNFα), early growth response-1 (egr-1), and plasminogen activator inhibitor-1 (PAI-1) were measured by real-time qRT-PCR.
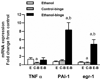
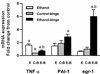
Results: Chronic ethanol treatment resulted in mild steatosis and necrosis, whereas chronic ethanol followed by binge group exhibited marked steatosis and significant increase in necrosis. Chronic binge group also showed significant increase (compared with chronic ethanol alone) in the phosphorylation of extracellular regulated kinase 1 (ERK1), ERK2, and RSK. Phosphorylation of c-Jun N-terminal kinase (JNK) and p38 MAPK did not increase by the binge. Ethanol binge, after chronic ethanol intake, caused increase in mRNA for egr-1 and PAI-1, but not TNFα.
Conclusions: Chronic ethanol exposure increases the susceptibility of rat liver to increased injury by 1 or 3 repeat binge. Among other alterations, the activated levels of ERK1, and more so ERK2, were remarkably amplified by binge suggesting a role of these isotypes in the binge amplification of the injury. In contrast, p38 MAPK and JNK1/2 activities were not amplified. These binge-induced changes were also reflected in the increases in the RNA levels for egr-1 and PAI-1. This study offers chronic followed by repeat binge as a model for the study of progression of liver injury by ethanol and highlights the involvement of ERK1 and ERK2 isotypes in the amplification of liver injury by binge ethanol.
Copyright © 2011 by the Research Society on Alcoholism.
Figures






References
-
- Adachi M, Brenner DA. Clinical syndromes of alcoholic liver disease. Dig Dis. 2005;23:255–263. - PubMed
-
- Amersi F, Shen Xiu-Da, Anselmo D, Anselmo D, Melinek J, Iyer S, Southard DJ, Katori M, Volk HD, Busuttil RW, Buelow R, Kupiec-Weglinski JW. Ex vivo exposure to carbon monoxide prevents hepatic ischemia /reperfusion injury through p38 MAP kinase pathway. Hepatology. 2002;35:815–823. - PubMed
-
- Anjum R, Blenis J. The RSK family of kinases: emerging roles in cellular signalling. Nat Rev Mol Cell Biol. 2008;9:747–758. - PubMed
-
- Apte M, McCarroll J, Pirola R, Wilson J. Pancreatic MAP kinase pathways and acetaldehyde. Novartis Found Symp. 2007;285:200–211. - PubMed
-
- Aroor AR, Custer GW, Weng YI, Lee YJ, Shukla SD. Phosphatidylethanol mimics ethanol modulation of p42/44 mitogen-activated protein kinase signalling in hepatocytes. Alcohol Alcohol. 2002;37:534–539. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

