Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice
- PMID: 21790939
- PMCID: PMC3196383
- DOI: 10.1111/j.1462-5822.2011.01646.x
Dissection of a type I interferon pathway in controlling bacterial intracellular infection in mice
Abstract
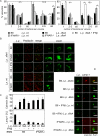
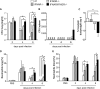
Defence mechanisms against intracellular bacterial pathogens are incompletely understood. Our study characterizes a type I IFN-dependent cell-autonomous defence pathway directed against Legionella pneumophila, an intracellular model organism and frequent cause of pneumonia. We show that macrophages infected with L. pneumophila produced IFNβ in a STING- and IRF3- dependent manner. Paracrine type I IFNs stimulated upregulation of IFN-stimulated genes and a cell-autonomous defence pathway acting on replicating and non-replicating Legionella within their specialized vacuole. Our infection experiments in mice lacking receptors for type I and/or II IFNs show that type I IFNs contribute to expression of IFN-stimulated genes and to bacterial clearance as well as resistance in L. pneumophila pneumonia in addition to type II IFN. Overall, our study shows that paracrine type I IFNs mediate defence against L. pneumophila, and demonstrates a protective role of type I IFNs in in vivo infections with intracellular bacteria.
© 2011 Blackwell Publishing Ltd.
Figures

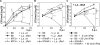

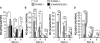
References
-
- Amer A, Franchi L, Kanneganti TD, Body-Malapel M, Ozoren N, Brady G, Meshinchi S, Jagirdar R, Gewirtz A, Akira S, Nunez G. Regulation of Legionella phagosome maturation and infection through flagellin and host Ipaf. J Biol Chem. 2006 17 11;281:35217–35223. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

