Improved functionality, health related quality of life and decreased burden of disease in patients with ADHD treated with OROS® MPH: is treatment response different between children and adolescents?
- PMID: 21791096
- PMCID: PMC3162502
- DOI: 10.1186/1753-2000-5-26
Improved functionality, health related quality of life and decreased burden of disease in patients with ADHD treated with OROS® MPH: is treatment response different between children and adolescents?
Abstract
Background: To compare clinical and health-related quality of life (HRQoL) outcomes between children and adolescents with ADHD treated with OROS® MPH, using data from two large similarly-designed multicenter, prospective, open-label, single-arm, non-interventional studies.
Methods: Pooled analysis (42603ATT4037, 42603 - ATT - 4001) including patients (6 to 18 years) with a confirmed diagnosis of ADHD. Patients were treated with OROS® MPH for 12 weeks; ADHD symptoms, functioning, HRQoL, safety and tolerability parameters were assessed.
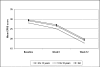
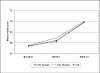
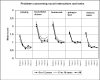
Results: 822 patients (583 children [6-12 years], 239 adolescents [13-18 years]) were included in the pooled analysis. Mean daily OROS® MPH starting doses in the child and adolescent subgroups were 29.0 ± 11.7 and 37.6 ± 15.6 mg, respectively (p < 0.001). At study end (week 12), the overall mean daily dose was 35.5 ± 14.0 mg, with children and adolescents receiving 32.8 ± 12.7 and 42.0 ± 15.1 mg/day, respectively (p < 0.001). Significant (p < 0.0001: overall population, children, adolescents) symptomatic, functional and HRQoL improvements were observed from baseline to study end using the Conners' Parents Rating Scale (overall: 29.2 ± 10.7 [baseline] to 19.3 ± 11.3 [endpoint]), Children's Global Assessment Scale (overall: 58.5 ± 14.5 [baseline] to 69.6 ± 16.1 [endpoint]), and ILC-LQ0-28. At week 12, between-age group differences were seen in the individual ILC-LQ0-28 parameters: school performance (p = 0.001 [parents' assessment], p = 0.032 [childrens' assessment]), global QoL (p = 0.012 [parents']) and interests and hobbies (p = 0.023 [childrens']). Treating physician's planned continued use of OROS® MPH in 76.9%, 86.0% and 79.3% of children, adolescents and the total population, respectively, at study end (p = 0.029 between-age subgroups). 195 of 822 patients (23.7%) experienced at least one treatment-emergent adverse event; most commonly reported AEs in the total group (≥4%) were insomnia (7.2%), anorexia (4.3%) and involuntary muscle contractions (4.1%). No clinically relevant changes in body weight or vital signs were observed.
Conclusions: Clinically relevant differences between children and adolescents with ADHD are present. Adolescents appeared to have a lower health related quality of life and functioning compared to children at baseline, however, they were able to reach comparable ratings at endpoint for most items. Similarly, burden of disease decreased in patients and their carers. OROS MPH was generally safe and well tolerated.
Figures


References
-
- Kooij SJ, Bejerot S, Blackwell A, Caci H, Casas-Brugué M, Carpentier PJ, Edvinsson D, Fayyad J, Foeken K, Fitzgerald M, Gaillac V, Ginsberg Y, Henry C, Krause J, Lensing MB, Manor I, Niederhofer H, Nunes-Filipe C, Ohlmeier MD, Oswald P, Pallanti S, Pehlivanidis A, Ramos-Quiroga JA, Rastam M, Ryffel-Rawak D, Stes S, Asherson P. European consensus statement on diagnosis and treatment of adult ADHD: The European Network Adult ADHD. BMC Psychiatry. 2010;10:67. doi: 10.1186/1471-244X-10-67. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

