Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection
- PMID: 21791421
- PMCID: PMC3179400
- DOI: 10.1182/blood-2011-04-347260
Gut inflammation and indoleamine deoxygenase inhibit IL-17 production and promote cytotoxic potential in NKp44+ mucosal NK cells during SIV infection
Abstract
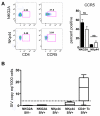
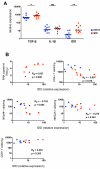
Natural killer (NK) cells are classically viewed as effector cells that kill virus-infected and neoplastic cells, but recent studies have identified a rare mucosal NK- cell subpopulation secreting the TH17 cytokine IL-22. Here, we report identification of 2 distinct lineages of mucosal NK cells characterized as NKG2A(+)NFIL3(+)RORC(-) and NKp44(+)NFIL3(+)RORC(+). NKG2A(+) NK cells were systemically distributed, cytotoxic, and secreted IFN-γ, whereas NKp44(+) NK cells were mucosae-restricted, noncytotoxic, and produced IL-22 and IL-17. During SIV infection, NKp44(+) NK cells became apoptotic, were depleted, and had an altered functional profile characterized by decreased IL-17 secretion; increased IFN-γ secretion; and, surprisingly, increased potential for cytotoxicity. NKp44(+) NK cells showed no evidence of direct SIV infection; rather, depletion and altered function were associated with SIV-induced up-regulation of inflammatory mediators in the gut, including indoleamine 2,3-dioxygenase 1. Furthermore, treatment of NKp44(+) NK cells with indoleamine 2,3-dioxygenase 1 catabolites in vitro ablated IL-17 production in a dose-dependent manner, whereas other NK-cell functions were unaffected. Thus lentiviral infection both depletes and modifies the functional repertoire of mucosal NK cells involved in the maintenance of gut integrity, a finding that highlights the plasticity of this rare mucosal NK-cell population.
Figures

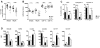
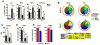
Similar articles
-
The secretion of IL-22 from mucosal NKp44⁺ NK cells is associated with microbial translocation and virus infection in SIV/SHIV-infected Chinese macaques.J Immunol Res. 2014;2014:387950. doi: 10.1155/2014/387950. Epub 2014 Dec 16. J Immunol Res. 2014. PMID: 25759828 Free PMC article.
-
Differential Effect of Mucosal NKp44+ Innate Lymphoid Cells and Δγ Cells on Simian Immunodeficiency Virus Infection Outcome in Rhesus Macaques.J Immunol. 2019 Nov 1;203(9):2459-2471. doi: 10.4049/jimmunol.1900572. Epub 2019 Sep 25. J Immunol. 2019. PMID: 31554692 Free PMC article.
-
IL-17-producing innate lymphoid cells are restricted to mucosal tissues and are depleted in SIV-infected macaques.Mucosal Immunol. 2012 Nov;5(6):658-69. doi: 10.1038/mi.2012.39. Epub 2012 Jun 6. Mucosal Immunol. 2012. PMID: 22669579 Free PMC article.
-
The Natural Cytotoxicity Receptors in Health and Disease.Front Immunol. 2019 May 7;10:909. doi: 10.3389/fimmu.2019.00909. eCollection 2019. Front Immunol. 2019. PMID: 31134055 Free PMC article. Review.
-
Incorporation of innate immune effector mechanisms in the formulation of a vaccine against HIV-1.Adv Exp Med Biol. 2011;780:143-59. doi: 10.1007/978-1-4419-5632-3_12. Adv Exp Med Biol. 2011. PMID: 21842371 Review.
Cited by
-
Relationships between IL-17(+) subsets, Tregs and pDCs that distinguish among SIV infected elite controllers, low, medium and high viral load rhesus macaques.PLoS One. 2013 Apr 19;8(4):e61264. doi: 10.1371/journal.pone.0061264. Print 2013. PLoS One. 2013. PMID: 23620737 Free PMC article.
-
Innate lymphoid cell interactions with microbiota: implications for intestinal health and disease.Immunity. 2012 Oct 19;37(4):601-10. doi: 10.1016/j.immuni.2012.10.003. Immunity. 2012. PMID: 23084357 Free PMC article. Review.
-
The initial interplay between HIV and mucosal innate immunity.Front Immunol. 2023 Jan 30;14:1104423. doi: 10.3389/fimmu.2023.1104423. eCollection 2023. Front Immunol. 2023. PMID: 36798134 Free PMC article. Review.
-
Simian Immunodeficiency Virus Infects Functionally Polarized Memory CD4 T Cells Equivalently In Vivo.J Virol. 2019 Apr 17;93(9):e02163-18. doi: 10.1128/JVI.02163-18. Print 2019 May 1. J Virol. 2019. PMID: 30787150 Free PMC article.
-
Orchestration of intestinal homeostasis and tolerance by group 3 innate lymphoid cells.Semin Immunopathol. 2018 Jul;40(4):357-370. doi: 10.1007/s00281-018-0687-8. Epub 2018 May 8. Semin Immunopathol. 2018. PMID: 29737384 Free PMC article. Review.
References
-
- Chinen H, Matsuoka K, Sato T, et al. Lamina propria c-kit+ immune precursors reside in human adult intestine and differentiate into natural killer cells. Gastroenterology. 2007;133(2):559–573. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

