Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient?
- PMID: 21791449
- PMCID: PMC3184240
- DOI: 10.1136/ard.2011.151043
Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient?
Abstract
Objective: To examine the risk of serious infection conveyed by tumour necrosis factor α (TNFα) inhibitors in the treatment of rheumatoid arthritis (RA).
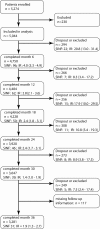
Methods: Data from patients with RA enrolled in the German biologics register RABBIT were used for analysis. Baseline patient characteristics, time-varying risk factors (treatment changes, functional capacity) and selection processes caused by dropout, death or switching to non-anti-TNF treatment were taken into account to estimate the adjusted incidence rate ratios (IRR(adj)) of serious infection during treatment with TNF inhibitors compared with non-biological disease-modifying antirheumatic drug treatment.
Results: Data were available on 5044 patients, in whom 392 serious infections occurred. The crude rates of serious infections in patients treated with TNF inhibitors declined over the first 3 years of observation (from 4.8 to 2.2/100 patient-years). This decline was driven by (1) treatment termination or loss to follow-up in patients at increased risk and (2) a risk reduction through decreasing glucocorticoid doses and improvement in function. Adjusted for selection processes and time-varying risk factors, the following parameters assessed at baseline (age, chronic diseases) or at follow-up prior to the infection were significantly associated with an increased risk: age >60 years, chronic lung or renal disease, low functional capacity, history of serious infections, treatment with glucocorticoids (7.5-14 mg/day, IRR(adj) 2.1 (95% CI 1.4 to 3.2); ≥ 15 mg/day, IRR(adj) 4.7 (95% CI 2.4 to 9.4)) and treatment with TNFα inhibitors (IRR(adj) 1.8 (95% CI 1.2 to 2.7)).
Conclusion: Reasons for the decline in infection rates observed at the group level were identified. The results enable expected infection rates to be calculated in individual patients based on their risk profiles.
Conflict of interest statement
Figures


Comment in
-
[Lung disease as infection risk].Z Rheumatol. 2013 Feb;72(1):4. Z Rheumatol. 2013. PMID: 23520634 German. No abstract available.
References
-
- Bongartz T, Sutton AJ, Sweeting MJ, et al. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. JAMA 2006;295:2275–85 - PubMed
-
- Leombruno JP, Einarson TR, Keystone EC. The safety of anti-tumour necrosis factor treatments in rheumatoid arthritis: meta and exposure-adjusted pooled analyses of serious adverse events. Ann Rheum Dis 2009;68:1136–45 - PubMed
-
- Listing J, Strangfeld A, Kary S, et al. Infections in patients with rheumatoid arthritis treated with biologic agents. Arthritis Rheum 2005;52:3403–12 - PubMed
-
- Dixon WG, Watson K, Lunt M, et al. Rates of serious infection, including site-specific and bacterial intracellular infection, in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Arthritis Rheum 2006;54:2368–76 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

