Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases
- PMID: 21791571
- PMCID: PMC8369516
- DOI: 10.1530/ERC-11-0144
Hepatic arterial infusion enhances DOTATOC radiopeptide therapy in patients with neuroendocrine liver metastases
Abstract
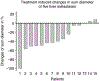
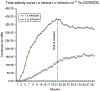
Intravenously administered radiolabeled peptides targeting somatostatin receptors are used for the treatment of unresectable gastroenteropancreatic neuroendocrine tumors (GEP-NETs). Recently, we demonstrated a high first-pass effect during intra-arterial (i.a.) administration of positron emission tomography (PET) labeled (68)Ga-DOTA(0)-d-Phe(1)-Tyr(3)-octreotide (DOTATOC). In this pilot study, we investigated the therapeutic effectiveness of arterial administered DOTATOC, labeled with the therapeutic β emitters (90)Y and (177)Lu. (90)Y- and/or (177)Lu-DOTATOC were infused into the hepatic artery of 15 patients with liver metastases arising from GEP-NETs. Response was assessed using DOTATOC-PET, multiphase contrast enhanced computed tomography, magnetic resonance imaging, and the serum tumor marker chromogranin A. Pharmacokinetic data of the arterial approach were assessed using (111)In-DOTATOC scans. With the treatment regime of this pilot study, complete remission was achieved in one (7%) patient and partial remission was observed in eight (53%) patients, six patients were classified as stable (40%; response evaluation criteria in solid tumors criteria). The concomitant decrease of elevated serum tumor marker confirmed the radiologic response. Median time to progression was not reached within a mean follow-up period of 20 months. Receptor saturation and redistribution effects were identified as limiting factors for i.a. DOTATOC therapy. The high rate of objective radiologic response in NET patients treated with arterial infusion of (90)Y-/(177)Lu-DOTATOC compares favorably with systemic chemotherapy and intravenous radiopeptide therapy. While i.a. DOTATOC therapy is only applicable to patients with tumors of limited anatomic distribution, the results of this pilot study are a promising development in the treatment of GEP-NET and warrants further investigation of this novel approach.
Conflict of interest statement
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Figures


References
-
- Bodei L, Cremonesi M, Ferrari M, Pacifici M, Grana CM, Bartolomei M, Baio SM, Sansovini M & Paganelli G 2008. Long-term evaluation of renal toxicity after peptide receptor radionuclide therapy with 90Y-DOTATOC and 177Lu-DOTATATE: the role of associated risk factors. European Journal of Nuclear Medicine and Molecular Imaging 35 1847–1856. (doi:10.1007/s00259-008-0778-1) - DOI - PubMed
-
- Bodei L, Pepe G & Paganelli G 2010. Peptide receptor radionuclide therapy (PRRT) of neuroendocrine tumors with somatostatin analogues. European Review for Medical and Pharmacological Sciences 14 347–351. - PubMed
-
- de Jong M, Breeman WA, Bernard BF, van Gameren A, de Bruin E, Bakker WH, van der Pluijm ME, Visser TJ, Mäcke HR & Krenning EP 1999. Tumour uptake of the radiolabelled somatostatin analogue (DOTA0, TYR3) octreotide is dependent on the peptide amount. European Journal of Nuclear Medicine 26 693–698. (doi:10.1007/s002590050439) - DOI - PubMed
-
- de Jong M, Breeman WA, Valkema R, Bernard BF & Krenning EP 2005. Combination radionuclide therapy using 177Lu- and 90Y-labeled somatostatin analogs. Journal of Nuclear Medicine 46 13S–17S. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

