Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential
- PMID: 21791601
- PMCID: PMC3165878
- DOI: 10.1104/pp.111.183210
Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential
Abstract
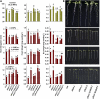
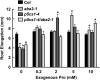
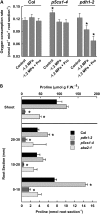
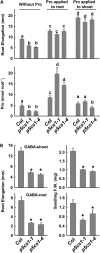
To better define the still unclear role of proline (Pro) metabolism in drought resistance, we analyzed Arabidopsis (Arabidopsis thaliana) Δ(1)-pyrroline-5-carboxylate synthetase1 (p5cs1) mutants deficient in stress-induced Pro synthesis as well as proline dehydrogenase (pdh1) mutants blocked in Pro catabolism and found that both Pro synthesis and catabolism were required for optimal growth at low water potential (ψ(w)). The abscisic acid (ABA)-deficient mutant aba2-1 had similar reduction in root elongation as p5cs1 and p5cs1/aba2-1 double mutants. However, the reduced growth of aba2-1 but not p5cs1/aba2-1 could be complemented by exogenous ABA, indicating that Pro metabolism was required for ABA-mediated growth protection at low ψ(w). PDH1 maintained high expression in the root apex and shoot meristem at low ψ(w) rather than being repressed, as in the bulk of the shoot tissue. This, plus a reduced oxygen consumption and buildup of Pro in the root apex of pdh1-2, indicated that active Pro catabolism was needed to sustain growth at low ψ(w). Conversely, P5CS1 expression was most highly induced in shoot tissue. Both p5cs1-4 and pdh1-2 had a more reduced NADP/NADPH ratio than the wild type at low ψ(w). These results indicate a new model of Pro metabolism at low ψ(w) whereby Pro synthesis in the photosynthetic tissue regenerates NADP while Pro catabolism in meristematic and expanding cells is needed to sustain growth. Tissue-specific differences in Pro metabolism and function in maintaining a favorable NADP/NADPH ratio are relevant to understanding metabolic adaptations to drought and efforts to enhance drought resistance.
Figures



References
-
- Abrahám E, Rigó G, Székely G, Nagy R, Koncz C, Szabados L. (2003) Light-dependent induction of proline biosynthesis by abscisic acid and salt stress is inhibited by brassinosteroid in Arabidopsis. Plant Mol Biol 51: 363–372 - PubMed
-
- Armengaud P, Thiery L, Buhot N, Grenier-De March G, Savouré A. (2004) Transcriptional regulation of proline biosynthesis in Medicago truncatula reveals developmental and environmental specific features. Physiol Plant 120: 442–450 - PubMed
-
- Bates LS, Waldren RP, Teare ID. (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39: 205–207
-
- Ben Hassine A, Ghanem ME, Bouzid S, Lutts S. (2008) An inland and a coastal population of the Mediterranean xero-halophyte species Atriplex halimus L. differ in their ability to accumulate proline and glycinebetaine in response to salinity and water stress. J Exp Bot 59: 1315–1326 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

