Human cancer-associated mutations in the Aα subunit of protein phosphatase 2A increase lung cancer incidence in Aα knock-in and knockout mice
- PMID: 21791616
- PMCID: PMC3165721
- DOI: 10.1128/MCB.05744-11
Human cancer-associated mutations in the Aα subunit of protein phosphatase 2A increase lung cancer incidence in Aα knock-in and knockout mice
Abstract
Strong evidence has indicated that protein phosphatase 2A (PP2A) is a tumor suppressor, but a mouse model for testing the tumor suppressor activity was missing. The most abundant forms of trimeric PP2A holoenzyme consist of the scaffolding Aα subunit, one of several regulatory B subunits, and the catalytic Cα subunit. Aα mutations were discovered in a variety of human carcinomas. All carcinoma-associated mutant Aα subunits are defective in binding the B or B and C subunits. Here we describe two knock-in mice expressing cancer-associated Aα point mutants defective in binding B' subunits, one knockout mouse expressing truncated Aα defective in B and C subunit binding, and a floxed mouse for generating conditional Aα knockouts. We found that the cancer-associated Aα mutations increased the incidence of cancer by 50 to 60% in lungs of FVB mice treated with benzopyrene, demonstrating that PP2A acts as a tumor suppressor. We show that the effect of Aα mutation on cancer incidence is dependent on the tumor suppressor p53. The finding that the Aα mutation E64D, which was detected in a human lung carcinoma, increases the lung cancer incidence in mice suggests that this mutation also played a role in the development of the carcinoma in which it was discovered.
Figures
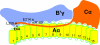




and and has no target in the Δ5-6 allele, the Δ5-6/Δ5-6 genotype yields no product (lane 5).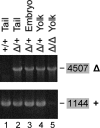
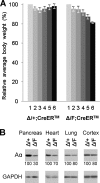

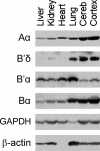

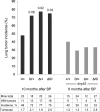

References
-
- Arroyo J. D., Hahn W. C. 2005. Involvement of PP2A in viral and cellular transformation. Oncogene 24:7746–7755 - PubMed
-
- Berger A. H., Pandolfi P. P. 2011. Haplo-insufficiency: a driving force in cancer. J. Pathol. 223:137–146 - PubMed
-
- Brewis N., et al. 2000. Dilated cardiomyopathy in transgenic mice expressing a mutant A subunit of protein phosphatase 2A. Am. J. Physiol. Heart Circ. Physiol. 279:H1307–H1318 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous
