Germline stem cells
- PMID: 21791699
- PMCID: PMC3220357
- DOI: 10.1101/cshperspect.a002642
Germline stem cells
Abstract
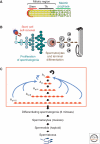
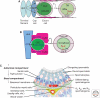
Sperm and egg production requires a robust stem cell system that balances self-renewal with differentiation. Self-renewal at the expense of differentiation can cause tumorigenesis, whereas differentiation at the expense of self-renewal can cause germ cell depletion and infertility. In most organisms, and sometimes in both sexes, germline stem cells (GSCs) often reside in a defined anatomical niche. Factors within the niche regulate a balance between GSC self-renewal and differentiation. Asymmetric division of the germline stem cell to form daughter cells with alternative fates is common. The exception to both these tendencies is the mammalian testis where there does not appear to be an obvious anatomical niche and where GSC homeostasis is likely accomplished by a stochastic balance of self-renewal and differentiation and not by regulated asymmetric cell division. Despite these apparent differences, GSCs in all organisms share many common mechanisms, although not necessarily molecules, to guarantee survival of the germline.
Figures
References
-
- Angelo G, Van Gilst MR 2009. Starvation protects germline stem cells and extends reproductive longevity in C. elegans. Science 326: 954–958 - PubMed
-
- Austin J, Kimble J 1987. glp-1 is required in the germline for regulation of the decision between mitosis and meiosis in C. elegans. Cell 51: 589–599 - PubMed
-
- Brawley C, Matunis E 2004. Regeneration of male germline stem cells by spermatogonial dedifferentiation in vivo. Science 304: 1331–1334 - PubMed
-
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE 2004. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet 36: 647–652 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
