Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids
- PMID: 21792174
- PMCID: PMC3160665
- DOI: 10.1038/emboj.2011.246
Distinct pathways for modification of the bacterial cell wall by non-canonical D-amino acids
Abstract
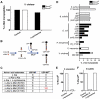
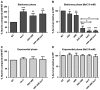
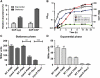
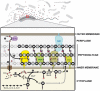
Production of non-canonical D-amino acids (NCDAAs) in stationary phase promotes remodelling of peptidoglycan (PG), the polymer that comprises the bacterial cell wall. Impairment of NCDAAs production leads to excessive accumulation of PG and hypersensitivity to osmotic shock; however, the mechanistic bases for these phenotypes were not previously determined. Here, we show that incorporation of NCDAAs into PG is a critical means by which NCDAAs control PG abundance and strength. We identified and reconstituted in vitro two (of at least three) distinct processes that mediate NCDAA incorporation. Diverse bacterial phyla incorporate NCDAAs into their cell walls, either through periplasmic editing of the mature PG or via incorporation into PG precursor subunits in the cytosol. Production of NCDAAs in Vibrio cholerae requires the stress response sigma factor RpoS, suggesting that NCDAAs may aid bacteria in responding to varied environmental challenges. The widespread capacity of diverse bacteria, including non-producers, to incorporate NCDAAs suggests that these amino acids may serve as both autocrine- and paracrine-like regulators of chemical and physical properties of the cell wall in microbial communities.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
-
- Aaron M, Charbon G, Lam H, Schwarz H, Vollmer W, Jacobs-Wagner C (2007) The tubulin homologue FtsZ contributes to cell elongation by guiding cell wall precursor synthesis in Caulobacter crescentus. Mol Microbiol 64: 938–952 - PubMed
-
- Barreteau H, Kovac A, Boniface A, Sova M, Gobec S, Blanot D (2008) Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol Rev 32: 168–207 - PubMed
-
- Biarrotte-Sorin S, Hugonnet JE, Delfosse V, Mainardi JL, Gutmann L, Arthur M, Mayer C (2006) Crystal structure of a novel beta-lactam-insensitive peptidoglycan transpeptidase. J Mol Biol 359: 533–538 - PubMed
-
- Bouhss A, Trunkfield AE, Bugg TD, Mengin-Lecreulx D (2008) The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol Rev 32: 208–233 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

