ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy
- PMID: 21792177
- PMCID: PMC3255596
- DOI: 10.1038/mt.2011.155
ACE2 and Ang-(1-7) confer protection against development of diabetic retinopathy
Abstract
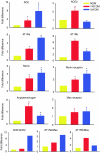
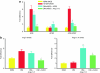
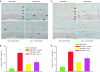
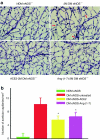
Despite evidence that hyperactivity of the vasodeleterious axis (ACE/angiotensin II (Ang II)/AT1 receptor) of the renin-angiotensin system (RAS) is associated with the pathogenesis of diabetic retinopathy (DR) use of the inhibitors of this axis has met with limited success in the control of this pathophysiology. We investigated the hypothesis that enhancing the local activity of the recently established protective axis of the RAS, ACE2/Ang-(1-7), using adeno-associated virus (AAV)-mediated gene delivery of ACE2 or Ang-(1-7) would confer protection against diabetes-induced retinopathy. Genes expressing ACE2 and Ang-(1-7) were cloned in AAV vector. The effects of ocular AAV-ACE2/Ang-(1-7) gene transfer on DR in diabetic eNOS(-/-) mice and Sprague-Dawley (SD) rats were examined. Diabetes was associated with approximately tenfold and greater than threefold increases in the ratios of ACE/ACE2 and AT1R/Mas mRNA levels in the retina respectively. Intraocular administration of AAV-ACE2/Ang-(1-7) resulted in significant reduction in diabetes-induced retinal vascular leakage, acellular capillaries, infiltrating inflammatory cells and oxidative damage in both diabetic mice and rats. Our results demonstrate that DR is associated with impaired balance of retinal RAS. Increased expression of ACE2/Ang-(1-7) overcomes this imbalance and confers protection against DR. Thus, strategies enhancing the protective ACE2/Ang-(1-7) axis of RAS in the eye could serve as a novel therapeutic target for DR.
Figures



References
-
- Cheung N, Mitchell P., and, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. - PubMed
-
- Mehta PK., and, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol, Cell Physiol. 2007;292:C82–C97. - PubMed
-
- Marchesi C, Paradis P., and, Schiffrin EL. Role of the renin-angiotensin system in vascular inflammation. Trends Pharmacol Sci. 2008;29:367–374. - PubMed
-
- de Cavanagh EM, Inserra F, Ferder M., and, Ferder L. From mitochondria to disease: role of the renin-angiotensin system. Am J Nephrol. 2007;27:545–553. - PubMed
-
- de Cavanagh EM, Ferder M, Inserra F., and, Ferder L. Angiotensin II, mitochondria, cytoskeletal, and extracellular matrix connections: an integrating viewpoint. Am J Physiol Heart Circ Physiol. 2009;296:H550–H558. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL102033/HL/NHLBI NIH HHS/United States
- HL56921/HL/NHLBI NIH HHS/United States
- UL1 TR000064/TR/NCATS NIH HHS/United States
- HL33610/HL/NHLBI NIH HHS/United States
- EY11123/EY/NEI NIH HHS/United States
- R01 HL033610/HL/NHLBI NIH HHS/United States
- R01 HL056921/HL/NHLBI NIH HHS/United States
- P30 EY021721/EY/NEI NIH HHS/United States
- R01 DK090730/DK/NIDDK NIH HHS/United States
- R01 EY011123/EY/NEI NIH HHS/United States
- R37 HL033610/HL/NHLBI NIH HHS/United States
- R01 HL110170/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

