Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma
- PMID: 21792199
- PMCID: PMC3170963
- DOI: 10.1038/bjc.2011.263
Targeting ErbB3-mediated stromal-epithelial interactions in pancreatic ductal adenocarcinoma
Abstract
Background: We sought to investigate the role of ErbB3-mediated signalling on the interaction between pancreatic cancer-associated fibroblasts (CAF) and carcinoma cells in an effort to disrupt tumourigenic pancreatic ductal adenocarcinoma (PDAC) stromal-epithelial cross-communication.
Methods: Primary CAF cultures were established from human PDAC surgical specimens. AsPC-1 pancreatic cancer cell murine subcutaneous xenografts were developed in the presence and absence of CAF and were subsequently treated with epidermal growth factor receptor (EGFR) inhibitors (erlotinib) and ErbB3 inhibitors (MM-121, monoclonal ErbB3 antibody).
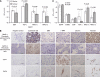
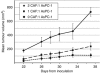
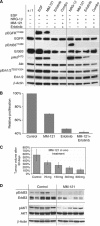
Results: Cancer-associated fibroblasts were found to secrete neuregulin-1 (NRG-1), which promoted proliferation via phosphorylation of ErbB3 and AKT in AsPC-1 PDAC cells. This signalling cascade was effectively inhibited both in vitro and in vivo by specific ErbB3 blockade with MM-121, with greater degree of tumourigenesis inhibition when combined with erlotinib. The CAF-AsPC-1 pancreatic cancer xenografts reached significantly greater tumour volume than those xenografts lacking CAF and were resistant to the anti-tumour effects of EGFR inhibition with erlotinib.
Conclusion: Cancer-associated fibroblasts-derived NRG-1 promote PDAC tumourigenesis via ErbB3-AKT signalling and overcomes single-agent EGFR inhibition. Disruption of this stromally mediated tumourigenic mechanism is best obtained through combined EGFR-ErbB3 inhibition with both erlotinib and MM-121. We have identified the NRG-1/ErbB3 axis as an attractive molecular target for the interruption of tumourigenic stromal-epithelial interactions within the PDAC microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



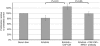


References
-
- Almoguera C, Shibata D, Forrester K, Martin J, Arnheim N, Perucho M (1988) Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53: 549–554 - PubMed
-
- Arnoletti JP, Frolov A, Eloubeidi M, Keene K, Posey J, Wood T, Greeno E, Jhala N, Varadarajulu S, Russo S, Christein J, Oster R, Buchsbaum DJ, Vickers SM (2011) A phase I study evaluating the role of the anti-epidermal growth factor receptor (EGFR) antibody cetuximab as a radiosensitizer with chemoradiation for locally advanced pancreatic cancer. Cancer Chemother Pharmacol 67: 891–897 - PMC - PubMed
-
- Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M (2008) Pancreatic stellate cells – role in pancreas cancer. Langenbecks Arch Surg 393: 891–900 - PubMed
-
- Berger MB, Mendrola JM, Lemmon MA (2004) ErbB3/HER3 does not homodimerize upon neuregulin binding at the cell surface. FEBS Lett 569: 332–336 - PubMed
-
- Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P (2009) Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol 27: 663–671 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

