Docetaxel and capecitabine for advanced gastric cancer: investigating dose-dependent efficacy in two patient cohorts
- PMID: 21792201
- PMCID: PMC3170974
- DOI: 10.1038/bjc.2011.278
Docetaxel and capecitabine for advanced gastric cancer: investigating dose-dependent efficacy in two patient cohorts
Abstract
Background: No comparisons of different doses of docetaxel-capecitabine in patients with advanced gastric cancer have been performed.
Methods: Patients with previously untreated metastatic/locally advanced gastro-oesophageal or gastric adenocarcinoma were enrolled in a prospective multicentre phase II trial. Two sequential cohorts received docetaxel 75 mg m(-2) (day 1) plus capecitabine 1000 mg m(-2) twice daily (days 1-14) (cohort I) or docetaxel 60 mg m(-2) (day 1) plus capecitabine 800 mg m(-2) twice daily (days 1-14) (cohort II) every 3 weeks. The primary end point was confirmed overall response rate.
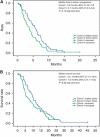
Results: In all, 91 patients were enrolled (cohort I, n=40; cohort II, n=51) and 87 were evaluable for efficacy (n=38, 49, respectively). Overall response rate was 50.0% in cohort I and 23.5% in cohort II (exploratory analysis, P=0.014). Median times to tumour progression and overall survival were 5.6 and 10.1 months in cohort I and 3.7 and 7.2 months in cohort II, respectively. Dose reductions for docetaxel and capecitabine were required in 50.0% and 57.5% of patients in cohort I and 11.8% and 15.7% in cohort II, respectively.
Conclusion: Starting treatment with full doses and reducing promptly seems to be the more promisingly effective strategy than starting cautiously with lower doses. Docetaxel/capecitabine 75/2000 mg m(-2) is a manageable, convenient outpatient combination with promising efficacy against advanced gastric cancer.
Figures
References
-
- Al-Batran SE, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, Stoehlmacher J, Clemens MR, Mahlberg R, Fritz M, Seipelt G, Sievert M, Pauligk C, Atmaca A, Jäger E (2008) Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol 19: 1882–1887 - PubMed
-
- Al-Batran S, Hohmann N, Hartmann JT, Moehler MH, Pauligk C, Probst S, Rethwisch V, Prasnikar N, Stoehlmacher J, Jaeger E (2010) 5-fluorouracil, leucovorin, and oxaliplatin with or without docetaxel in elderly (65 years or older) patients with esophagogastric cancer: FLOT65+ trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO). J Clin Oncol 28(Suppl): 15s (abstract 4013)
-
- Chau I, Norman AR, Cunningham D, Oates J, Hawkins R, Iveson T, Nicolson M, Harper P, Seymour M, Hickish T (2009) The impact of primary tumour origins in patients with advanced oesophageal, oesophago-gastric junction and gastric adenocarcinoma – individual patient data from 1775 patients in four randomised controlled trials. Ann Oncol 20: 885–891 - PubMed
-
- Chun JH, Kim HK, Lee JS, Choi JY, Hwangbo B, Lee HG, Park SR, Choi IJ, Kim CG, Ryu KW, Kim YW, Lee JS, Bae JM (2005) Weekly docetaxel in combination with capecitabine in patients with metastatic gastric cancer. Am J Clin Oncol 28: 188–194 - PubMed
-
- Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, Middleton G, Daniel F, Oates J, Norman AR, Upper Gastrointestinal Clinical Studies Group of the National Cancer Research Institute of the United Kingdom (2008) Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med 358: 36–46 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

