Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α
- PMID: 21792242
- PMCID: PMC3323291
- DOI: 10.1038/jcbfm.2011.106
Tissue-type plasminogen activator has a neuroprotective effect in the ischemic brain mediated by neuronal TNF-α
Abstract
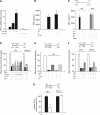
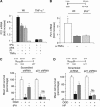
Cerebral cortical neurons have a heightened sensitivity to hypoxia and their survival depends on their ability to accommodate to changes in the concentration of oxygen in their environment. Tissue-type plasminogen activator (tPA) is a serine proteinase that activates the zymogen plasminogen into plasmin. Hypoxia induces the release of tPA from cerebral cortical neurons, and it has been proposed that tPA mediates hypoxic and ischemic neuronal death. Here, we show that tPA is devoid of neurotoxic effects and instead is an endogenous neuroprotectant that renders neurons resistant to the effects of lethal hypoxia and ischemia. We present in vitro and in vivo evidence indicating that endogenous tPA and recombinant tPA induce the expression of neuronal tumor necrosis factor-α. This effect, mediated by plasmin and the N-methyl-D-aspartate receptor, leads to increased expression of the cyclin-dependent kinase inhibitor p21 and p21-mediated development of early hypoxic and ischemic tolerance.
Figures
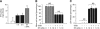
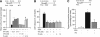


References
-
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
-
- Baron A, Montagne A, Casse F, Launay S, Maubert E, Ali C, Vivien D. NR2D-containing NMDA receptors mediate tissue plasminogen activator-promoted neuronal excitotoxicity. Cell Death Differ. 2010;17:860–871. - PubMed
-
- Barone FC, Arvin B, White RF, Miller A, Webb CL, Willette RN, Lysko PG, Feuerstein GZ. Tumor necrosis factor-alpha. A mediator of focal ischemic brain injury. Stroke. 1997;28:1233–1244. - PubMed
-
- Belayev L, Busto R, Zhao W, Fernandez G, Ginsberg MD. Middle cerebral artery occlusion in the mouse by intraluminal suture coated with poly-L-lysine: neurological and histological validation. Brain Res. 1999;833:181–190. - PubMed
-
- Benchenane K, Berezowski V, Ali C, Fernandez-Monreal M, Lopez-Atalaya JP, Brillault J, Chuquet J, Nouvelot A, MacKenzie ET, Bu G, Cecchelli R, Touzani O, Vivien D. Tissue-type plasminogen activator crosses the intact blood-brain barrier by low-density lipoprotein receptor-related protein-mediated transcytosis. Circulation. 2005;111:2241–2249. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

