Quercetin and cancer chemoprevention
- PMID: 21792362
- PMCID: PMC3136711
- DOI: 10.1093/ecam/neq053
Quercetin and cancer chemoprevention
Abstract
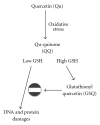
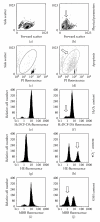
Several molecules present in the diet, including flavonoids, can inhibit the growth of cancer cells with an ability to act as "chemopreventers". Their cancer-preventive effects have been attributed to various mechanisms, including the induction of cell-cycle arrest and/or apoptosis as well as the antioxidant functions. The antioxidant activity of chemopreventers has recently received a great interest, essentially because oxidative stress participates in the initiation and progression of different pathological conditions, including cancer. Since antioxidants are capable of preventing oxidative damage, the wide use of natural food-derived antioxidants is receiving greater attention as potential anti-carcinogens. Among flavonoids, quercetin (Qu) is considered an excellent free-radical scavenging antioxidant, even if such an activity strongly depends on the intracellular availability of reduced glutathione. Apart from antioxidant activity, Qu also exerts a direct, pro-apoptotic effect in tumor cells, and can indeed block the growth of several human cancer cell lines at different phases of the cell cycle. Both these effects have been documented in a wide variety of cellular models as well as in animal models. The high toxicity exerted by Qu on cancer cells perfectly matches with the almost total absence of any damages for normal, non-transformed cells. In this review we discuss the molecular mechanisms that are based on the biological effects of Qu, and their relevance for human health.
Figures

References
-
- Danaei G, Vander Hoorn S, Lopez AD, Murray CJL, Ezzati M. Causes of cancer in the world: comparative risk assessment of nine behavioural and environmental risk factors. Lancet. 2005;366(9499):1784–1793. - PubMed
-
- Knekt P, Kumpulainen J, Järvinen R, et al. Flavonoid intake and risk of chronic diseases. American Journal of Clinical Nutrition. 2002;76(3):560–568. - PubMed
-
- Manson MM. Cancer prevention—the potential for diet to modulate molecular signalling. Trends in Molecular Medicine. 2003;9(1):11–18. - PubMed
-
- Van Duijnhoven FJB, Bueno-De-Mesquita HB, Ferrari P, et al. Fruit, vegetables, and colorectal cancer risk: the European Prospective Investigation into Cancer and Nutrition. American Journal of Clinical Nutrition. 2009;89(5):1441–1452. - PubMed

