Immunohistochemical characterization of human olfactory tissue
- PMID: 21792956
- PMCID: PMC3181071
- DOI: 10.1002/lary.21856
Immunohistochemical characterization of human olfactory tissue
Abstract
Objectives/hypothesis: The pathophysiology underlying human olfactory disorders is poorly understood because biopsying the olfactory epithelium (OE) can be unrepresentative and extensive immunohistochemical analysis is lacking. Autopsy tissue enriches our grasp of normal and abnormal olfactory immunohistology and guides the sampling of the OE by biopsy. Furthermore, a comparison of the molecular phenotype of olfactory epithelial cells between rodents and humans will improve our ability to correlate human histopathology with olfactory dysfunction.
Study design: An immunohistochemical analysis of human olfactory tissue using a comprehensive battery of proven antibodies.
Methods: Human olfactory mucosa obtained from 21 autopsy specimens was analyzed with immunohistochemistry. The position and extent of olfactory mucosa was assayed by staining whole mounts (WMs) with neuronal markers. Sections of the OE were analyzed with an extensive group of antibodies directed against cytoskeletal proteins and transcription factors, as were surgical specimens from an esthesioneuroblastoma.
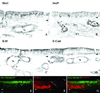
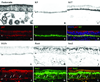
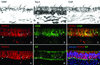
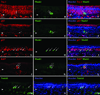
Results: Neuron-rich epithelium is always found inferior to the cribriform plate, even at advanced age, despite the interruptions in the neuroepithelial sheet caused by patchy respiratory metaplasia. The pattern of immunostaining with our antibody panel identifies two distinct types of basal cell progenitors in human OE similar to rodents. The panel also clarifies the complex composition of esthesioneuroblastoma.
Conclusions: The extent of human olfactory mucosa at autopsy can easily be delineated as a function of age and neurologic disease. The similarities in human versus rodent OE will enable us to translate knowledge from experimental animals to humans and will extend our understanding of human olfactory pathophysiology.
Copyright © 2011 The American Laryngological, Rhinological, and Otological Society, Inc.
Conflict of interest statement
Financial Disclosure: None of the authors have any financial interest in companies or other entities that may have an interest in this work. There are no conflicts of interest to disclose for any of the authors.
Figures


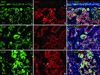
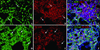

References
-
- Naessen R. The identification and topographical localisation of the olfactory epithelium in man and other mammals. Acta Otolaryngol. 1970;70:51–57. - PubMed
-
- Nakashima T, Kimmelman CP, Snow JB., Jr Structure of human fetal and adult olfactory neuroepithelium. Arch Otolaryngol. 1984;110:641–646. - PubMed
-
- Paik SI, Lehman MN, Seiden AM, Duncan HJ, Smith DV. Human olfactory biopsy. The influence of age and receptor distribution. Arch Otolaryngol Head Neck Surg. 1992;118:731–738. - PubMed
-
- Hasegawa S, Yamagishi M, Nakano Y. Microscopic studies of human olfactory epithelia following traumatic anosmia. Arch Otorhinolaryngol. 1986;243:112–116. - PubMed
-
- Holbrook EH, Leopold DA, Schwob JE. Abnormalities of axon growth in human olfactory mucosa. Laryngoscope. 2005;115:2144–2154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

